
Consider our favorite reaction:
a A(g) + b B(g) → ← c C(g) + d D(g)
The equilibrium constant mass action expression is:
What does it mean if Kc is a large number?
If Kc is a large number, [C]e and [D]e must be large and [A]e and [B]e must be small. Chemically, this means that the reaction favors products at equilibrium (or, using a common expression, that the equilibrium favors the products).
What does it mean if Kc small number?
If Kc is a small number, [C]e and [D]e must be small and [A]e and [B]e must be large. Chemically, this means that the reaction favors reactants at equilibrium (or that the equilibrium favors the reactants).
Suppose the mass action expression is evaluated at nonequilibrium conditions:
Qc is called the reaction quotient: the evaluation of the mass action expression at any set of conditions.
Qc is not a constant but it can be used to determine the direction a reaction will proceed in order to reach equilibrium.
If Qc > Kc, then the numerator (product concentrations) is too large and the denominator (reactant concentrations) is too small; in order to reach equilibrium the reaction must proceed from products to reactants; i.e. to the left.
If Qc < Kc, then the numerator (product concentrations) is too small and the denominator (reactant concentrations) is too large; in order to reach equilibrium the reaction must proceed from reactants to products; i.e. to the right.
If Qc = Kc, then the reaction is at equilibrium and no macroscopic change in concentrations can be observed.
For the reaction of hydrogen with nitrogen to form ammonia, the equilibrium constant Kc = 4.1×108 at 25 °C. A reaction was run and the equilibrium concentrations were found to be 1.0×10–3 M in both hydrogen and nitrogen and 0.020 M in ammonia. 0.010 M nitrogen was added. Which direction does the reaction shift?
Strategy: write the reaction, write the mass action expression, calculate the reaction quotient, and then compare Qc to Kc.
The reaction:
N2(g) + 3 H2(g) → ← 2 NH3(g)
The mass action expression is
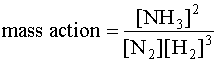
After the nitrogen is added, the concentrations of species are
[N2] = 1.0×10–3 + 0.010 = 0.011 M
[H2] = 1.0×10–3 M
[NH3] = 0.020 M
So the reaction quotient is
3.6×107<4.1×108 (i.e. Qc<Kc) so the reaction must proceed toward products: adding the nitrogen will cause more ammonia to be created.
When a stress (i.e., a change in composition, temperature, pressure, etc.) is applied to a system at equilibrium, the system will reestablish equilibrium in a manner that alleviates the applied stress.
In the above example, addition of nitrogen to the equilibrium (the stress) caused the reaction to proceed towards products; chemically, this removed some of the added nitrogen (alleviated the stress).
In general:
If a component is added to a reaction, the reaction shifts to remove the added component.
If a component is removed from a reaction, the reaction shifts to replace that component.
If the partial pressure of a component is increased, the reaction shifts to reduce the pressure.
If the partial pressure of a component is reduced, the reaction shifts to increase the pressure.
If the temperature is increased, the reaction shifts to remove the added heat (i.e., toward the endothermic direction).
If the temperature is decreased, the reaction shifts to replace the heat (i.e., toward the exothermic direction).
The following reaction is allowed to come to equilibrium.
N2O3(g) → ← NO(g) + NO2(g)
ΔH° = +39.7 kJ/mol
Which way does the reaction shift if:
The total pressure is increased by reducing the volume of the reaction vessel?