
Since the formation of the activated complex is the most important feature in determining the rate of a reaction, the temperature dependence of the collision frequency is often ignored. Then, the rate constant can be written as
k is the rate constant for the reaction
Ea is the activation energy
R is the gas constant in energy units = 8.314 J/mol·K
T is the absolute temperature
A is the pre-exponential factor.
The Arrhenius equation is often written in the logarithmic form:
This is (yet another) straight line equation: if ln k is plotted against 1/T, a straight line is found. The slope of the line gives the activation energy:
slope = –Ea/R
To obtain activation energies, the rate constant is measured at several different temperatures. These are then plotted as ln k vs. 1/T and the slope of the straight line gives the activation energy.
Consider the reaction:
2 HI(g) → H2(g) + I2(g)
The following data was measured:
k ln k T (oC) T (K) 1/T
3.52×10–7 –14.860 283 556 1.80×10–3
3.02×10–5 –10.408 356 629 1.59×10–3
2.19×10–4 –8.426 393 666 1.50×10–3
1.16×10–3 –6.759 427 700 1.43×10–3
3.95×10–2 –3.231 508 781 1.28×10–3
If there is a limited amount of data, the two-point form of the Arrhenius equation can be used:
If we know the rate constants at two temperatures, then the activation energy can be found. This gives less accurate values for Ea, but is computationally quicker.
Find the activation energy for the following reaction:
CO(g) + NO2(g) → CO2(g) + NO(g)
The rate constants were found to be 0.028 M–1s–1 at 327 oC and 23 M–1s–1 at 527 oC.
k1 = 0.028 M–1s-1 and T1 = 327 + 273 = 600 K
k2 = 23 M–1s–1 and T2 = 527 + 273 = 800 K
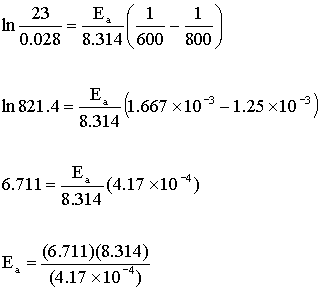
Ea = 133800 J/mol = 130 kJ/mol
What is the total order of reaction?
Reactions are caused by collisions between molecules. Thus, we should be able to describe every reaction, no matter how complicated, by a series of collisions. This is called a reaction mechanism.
An elementary reaction is a reaction that describes a physical collision at the microscopic level.
The sequence of elementary reactions that lead to a macroscopic reaction is called the reaction mechanism.
Reaction mechanisms are just a list of elementary reactions in the correct order.
Elementary reactions are special because the rate law can be found from the stoichiometric coefficients: the order of reaction in each reactant is equal to its stoichiometric coefficient.
Remember that this is true only for elementary reactions.