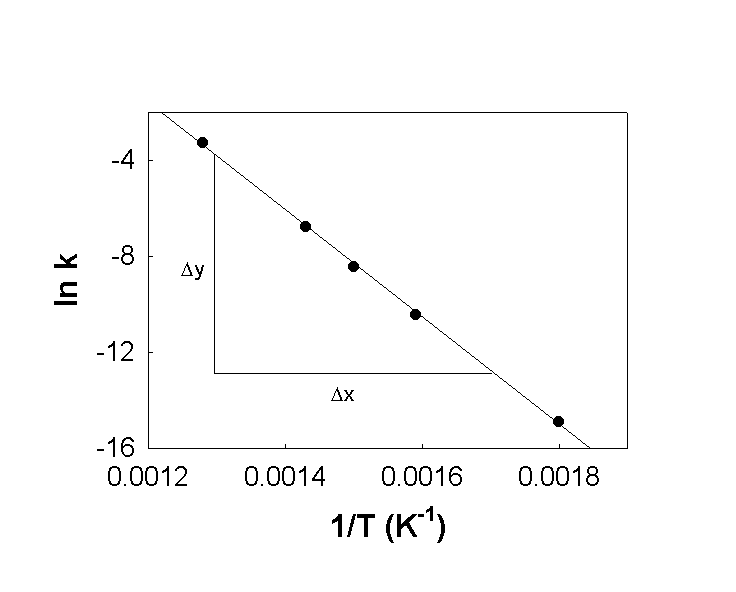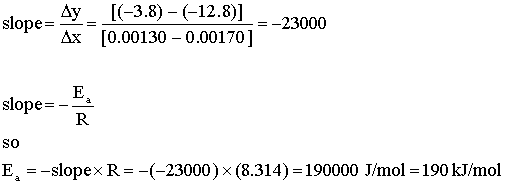To answer the question, we need to find ln k, T in K, 1/T, and then plot ln k vs. 1/T. The slope of the line will lead us to the activation energy.
k ln k T (oC) T (K) 1/T
3.52×10–7 –14.860 283 556 1.80×10–3
3.02×10–5 –10.408 356 629 1.59×10–3
2.19×10–4 –8.426 393 666 1.50×10–3
1.16×10–3 –6.759 427 700 1.43×10–3
3.95×10–2 –3.231 508 781 1.28×10–3
Plot: