
Consider the reaction:
BrO3–(aq) + 5 Br–(aq) + 6 H +(aq) →3 Br2(aq) + 3 H2O(l)
The following data was obtained:
Experiment #
[BrO3–] (M)
[Br–] (M)
[H+] (M)
Initial rate (M s–1)
1
0.10
0.10
0.10
–1.2×10–3
2
0.20
0.10
0.10
–2.4×10–3
3
0.20
0.30
0.10
–7.4×10–3
4
0.20
0.10
0.15
–5.4×10–3
From the definition of the rate law we can write:
Rate = k[BrO3–]m[Br –]n[H+]p
How do we find the order of reaction in bromate ion?
How do we find the order of reaction in bromide ion?
How do we find the reaction order in hydrogen ion?
Finally, we need to find the rate constant k:
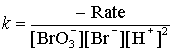
(Why is there a negative sign added?)
So for each experiment:
Experiment # k (L3/mol3s–1)
1
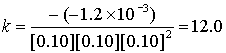
2
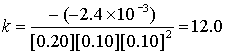
3
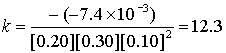
4
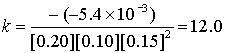
The average is k = 12 L3/mol3s–1
So the final rate law is
Rate = k[BrO3–][Br–][H+]2
with k = 12 L3/mol3s–1
The rate law can tell us the rate of reaction at any time and with any concentration of reactants. We would also like to know the concentration of reactants or products after a certain passage of time if we know the initial concentration of reactants.
The rate law also gives us this information, but it does involve solving differential equations – we don't need to know how this is done but we do need to be familiar with some simple results.
These are known as Integrated Rate Laws
A → products
Rate = –k[A]0 = –k
After solving the math:
[A] = –kt + [A]0
where [A]0 is the initial concentration of the reactant
A plot of [A] vs. time is linear:
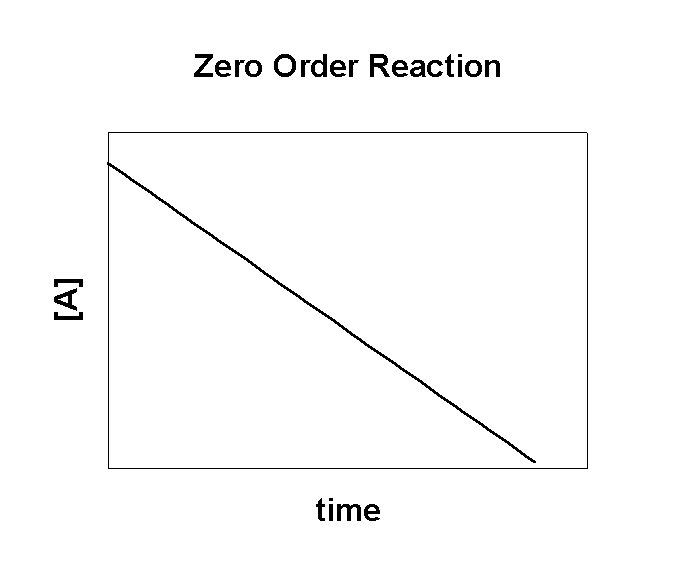
The slope of the line gives the rate constant:
k = –slope
A → products
Rate = –k[A]
After solving the math:
where [A]0 is the initial concentration of the reactant
A plot of ln[A] (or log[A]) vs. time is linear:
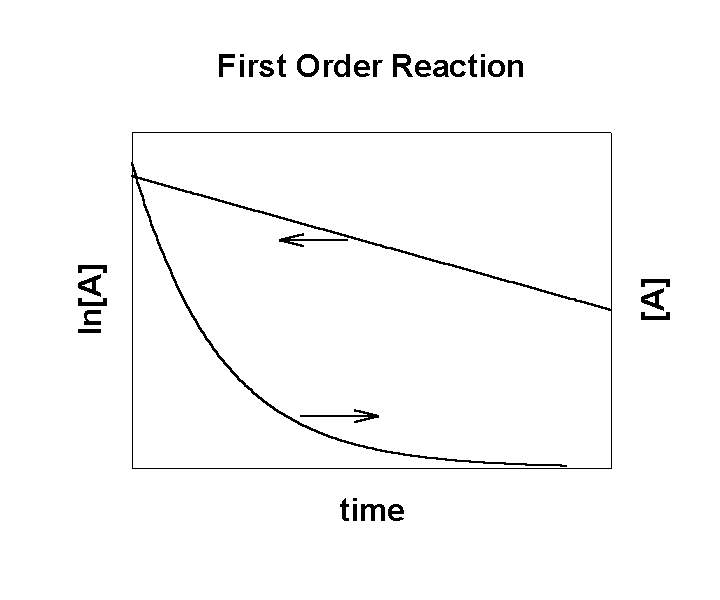
The slope of the line for the plot of ln[A] vs. t gives the rate constant:
k = –slope
A → products
Rate = –k[A]2
After solving the math:
where [A]0 is the initial concentration of the reactant
A plot of 1/[A] vs. time is linear:
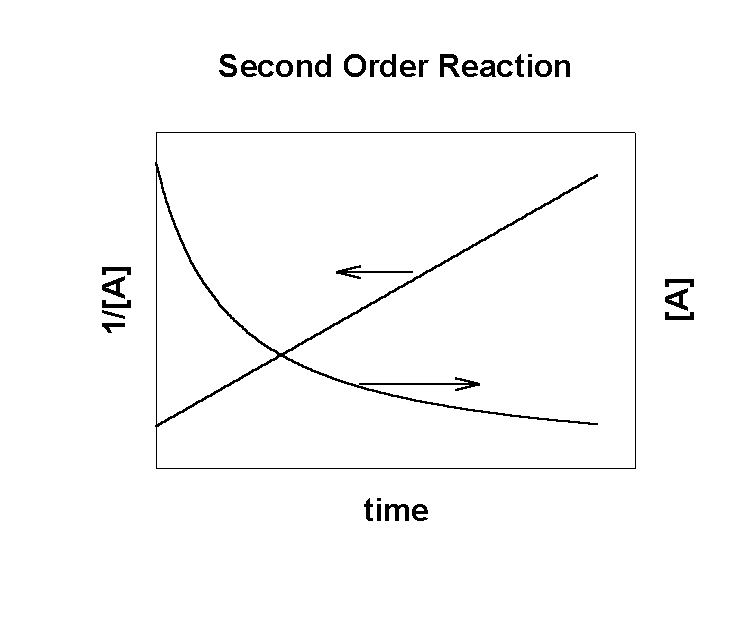
The plot of 1/[A] vs. t gives the rate constant:
slope = +k
Now we have a new method of establishing the rate law. If concentration data is collected as a function of time, then by finding the linear plot we have found the order of reaction and the rate constant.