
Experiment # [BrO3–] (M) [Br–] (M) [H+] (M) Initial rate (M s–1)
1 0.10 0.10 0.10 –1.2×10–3
2 0.20 0.10 0.10 –2.4×10–3
3 0.20 0.30 0.10 –7.4×10–3
4 0.20 0.10 0.15 –5.4×10–3
Experiments 2 and 4 can be used to give the order in hydrogen ion:
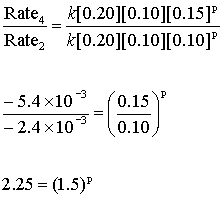
So here, p = 2
The reaction is second order in hydrogen ion.
(if this isn't obvious, a more rigorous mathematical solution is found here)
Back