
- Daniel Thomas
- Beaupre 474D
- Assistant Professor
- (401) 874-5834
- Ph.D. California Institute of Technology
- dathomas@uri.edu
Our research group’s overall focus is to leverage the selectivity and control afforded under vacuum conditions to interrogate species that are dynamic, transient, or otherwise difficult to isolate in solution. We apply this approach to investigate topics that span chemistry research areas, including biomolecular structure and dynamics, mechanisms in homogeneous catalysis, structural motifs in deep eutectic solvents, and chemistry of the liquid/vapor interface.
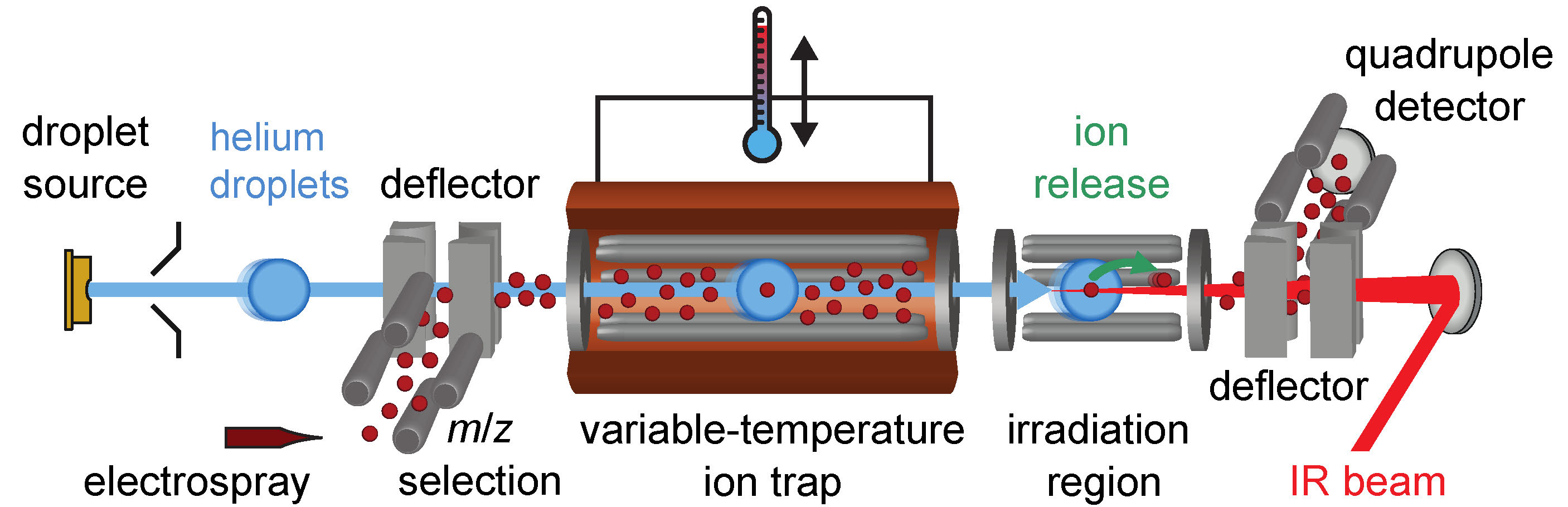
To carry out this research, we develop custom instrumentation that combines the gentle ionization and selectivity afforded by electrospray ionization mass spectrometry (ESI–MS) with the structural insight available from infrared action spectroscopy. As illustrated in Figure 1, ions of interest are introduced to the instrument using a standard atmospheric pressure interface and are then selected by m/z using a quadrupole mass filter. The selected ions are then transferred to a variable-temperature ion trap, where trap temperature is modulated by a flow of nitrogen through a copper housing surrounding the trap. The structure of the trapped ions can either be probed directly using infrared photodissociation (IRPD) action spectroscopy or by helium nanodroplet capture and subsequent spectroscopic interrogation. In IRPD action spectroscopy, ion dissociation is initiated by the sequential absorption of multiple resonant infrared photons, and the yield of dissociated ions is monitored as a function of incident photon energy to obtain an IR action spectrum.
In addition to characterization by IRPD action spectroscopy, ions can be interrogated by helium nanodroplet isolation infrared (HENDI IR) action spectroscopy. This method represents a powerful technique for the experimental investigation of the structure and properties of molecules and clusters. Although HENDI IR spectroscopy has long been applied to the interrogation of neutral species, the ability to readily perform such experiments on molecular ions has been developed only recently. Following the accumulation of ions in the hexapole trap, a beam of helium nanodroplets, generated by the expansion of cold, high-pressure helium into vacuum, traverses the trap, resulting in ion pickup (see Figure 1). The nanodroplets with entrained ions then enter an IR irradiation region, where the absorption of multiple resonant photons results in the production of bare ions. These bare ions are then deflected to an off-axis quadrupole mass filter for detection. The ion signal as a function of the incident photon energy is used as an action signal to generate an infrared spectrum.
More information about our research projects can be found on the research page. A CV for Prof. Thomas and a link to Google Scholar can be found at the lower right of this page.
