Clusters and Rugged Energy Landscapes
Clusters are small aggregates of atoms and/or molecules, and owing to their central role in nucleation phenomena, clusters are widely studied both experimentally and theoretically. Typically materials are referred to as clusters when the material consists of tens to several thousand monomer units. Because of their small size and the predominance of surface interactions, the properties and structures of clusters frequently differ significantly from the corresponding bulk material. For example, solid argon forms an hcp lattice, where as thirteen argon atoms form an icosahedral structure displayed here
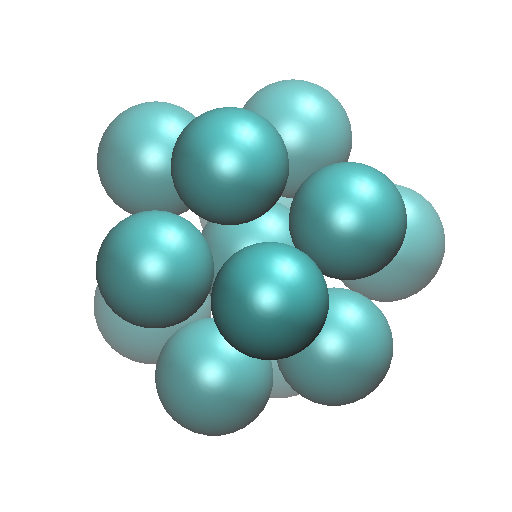
Each vertex in the figure represents an argon atom, and the structure has a five fold symmetry rather than the closest packed hcp structure that is observed in bulk, solid argon. Because nucleation leading to crystal growth begins by monomer addition onto small clusters, a key question concerns how the bulk hcp structure evolves from small nuclei having pentagonal rather than closest-packed symmetry.
Because clusters have structures and properties that differ significantly from the corresponding bulk materials, and important issue has been the thermodynamic properties of clusters, and how the properties evolve with cluster size. The thermodynamic properties of clusters reflect the structure of the underlying potential energy surface of the aggregate, and such underlying potential energy surface have remarkable complexities that are reminiscent of the complexities seen in other materials like proteins. The heat capacity of a 38-particle argon cluster shown here
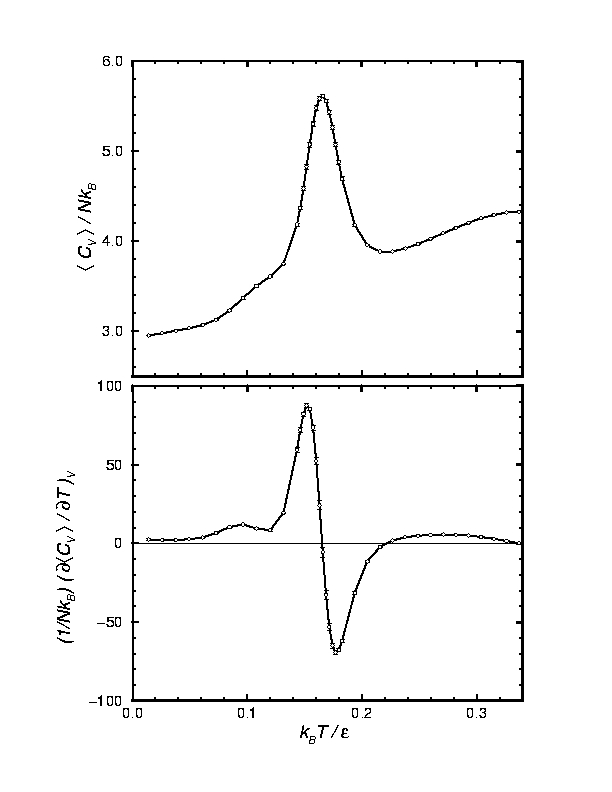
is a good example of the reflection of the potential surface on properties. The potential surface of the 38-atom cluster has a complex double-funnel structure with high energy barriers separating two distinct basins. The low-temperature shoulder of the heat capacity observable as the small peak in the temperature derivative corresponds to solid-like to solid-like phase changes in the system. Owing to the complex potential surface, the computation of the heat capacity curve requires enhanced sampling techniques, and such methodological developments are an important part of our research.
