Posts in Category: Presentations


Two posters presented at the 250th National ACS meeting in Boston, MA
ORGN: Division of Organic Chemistry
523 – Regioselective C–N bond formation via gold catalysis
Louis Marchetti1 , Abhishek Kantak1 , Riley Davis1 , h_davis@my.uri.edu, Brenton DeBoef1
1 University of Rhode Island, Kingston, Rhode Island, United States
Abstract:This poster presents a novel protocol for the intermolecular, regioselective amination of arenes. A direct and novel route for regioselectively accessing various substituted aniline compounds has been achieved through the use of an Iodine (III) oxidant in the presence of a Gold (I) catalyst. Yields as high as 90% were recorded. Regioselectivity can be predicted based on electrophilic aromatic metalation patterns.

ORGN: Division of Organic Chemistry
477 – Binding analysis of Xe-129 with cyclotriveratrylenes (CTV) compounds
John Rhoat1 , john.rhoat008@gmail.com
1 Chemistry, University Of Rhode Island, Kingston, Rhode Island, United States
Abstract:An amino functionalized cyclotriveratrylene (CTV), a bowl-shaped molecule, was recently shown to reversibly bind Xe-129 through unique chemical shift in the 129Xe NMR spectrum. The nature of the host guest relationship between the amine CTV and the Xe-129 is largely unknown. To explore this relationship and investigate the hypothesis of hydrogen bonding around Xe-129 to form a cryptophane like cage, the substituents around the rim of the bowl were varied. Several synthesized derivatives were tested in their ability to bind Xe-129.


Two posters presented at the 8th Annual RI SURF Conference
Organic Chemistry
The 8th Annual Rhode Island Summer Undergraduate Research Fellows Conference
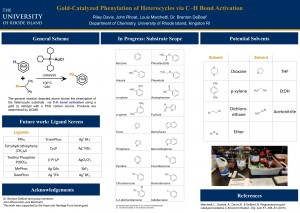
Gold-Catalyzed Phenylation of Heterocycles via C–H Bond Activation
Riley Davis1, John Rhoat1, Louis Marchetti1, Brenton DeBoef1
1 University of Rhode Island, Kingston, Rhode Island, United States
Abstract: This research aims for the regioselective, intermolecular addition of C-C bonds to heterocycles such as pyrroles, thiophenes, and furans, via C-H bond activation. Using a novel I(III) phenyl-source in the presence of a Au(I) catalyst results in a greener and more direct synthetic route for regioselectively accessing a variety of biarly compounds. This is exemplified by the successful synthesis of three heterocyclic biaryls. Further studies hope to increase yields, prove a wider scope of application, and elucidate the mechanism of this novel reaction.
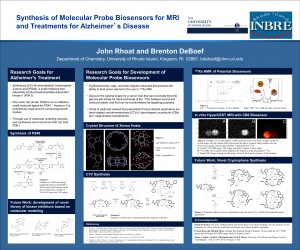
Synthesis of Molecular Probe Biosensors for MRI and Treatments for Alzheimer’s Disease
John Rhoat1, Brenton DeBoef1
1 University of Rhode Island, Kingston, Rhode Island, United States
Abstract: A cyclotriveratrylene (CTV) compound has been identified as a novel molecular probe for 129Xe magnetic resonance imaging (MRI) and spectroscopy. Past work in this field has relied on the use of Cryptophanes, cage shaped molecules whose syntheses are difficult and low yielding. CTV is a bowl-shaped compound that is also capable of reversibly binding xenon; this event can be detected in 129Xe NMR spectrum. Due to the ease of synthesis of this novel CTV, we hypothesize that it can be a superior molecular probe for functionalization and eventual use as a targeted 129Xe molecular probe.
PS48, (Z)-5-(4-chlorophenyl)-3-phenylpent-2-enoic acid, is known to bind phosphoinositide-dependent protein kinase 1 (PDK1a ) which is involved in insulin signaling pathways. The complete pathogenesis of Alzheimer’s disease (AD) is still unknown, but one emerging hypothesis is it may be a “type III diabetes,” due to the association of insulin depletion in the brain with early stages of AD. Synthesis of PS48 has been achieved in our lab in 4 steps. We hope to use this compound as a key component in a collaborative effort towards the development of a library of novel kinase inhibitors for potential treatment of AD.