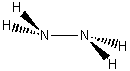 d)
d) 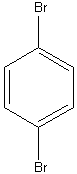 e)
e)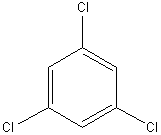
Answers
November 2, 2000
Open book, open notes
1. Find the point group for the following molecules:
a) HF b) CF3H c)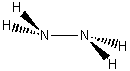 d)
d)  e)
e)
Answers
2. Construct a molecular orbital energy diagram for Cr2 using only the 3d orbitals as the basis set. Label each orbital as nb, σ, π, δ, σ*, π*, or δ*. Show that the bond order is equal to 4. How many unpaired spins are there? Sketch the shapes and phase relationships of each of the bonding orbitals; if there are degenerate orbitals, only sketch one orbital of that energy. Hint: although the 4s orbital in Cr is not used in the basis set, the electrons from the 4s orbitals are used in the MO diagram.
3. Find the irreducible representations for the σ bonds in NH3.
4. Find the irreducible representations of the C π
orbitals in ![]() .
Draw sketches of each of the orbitals and energy order them. Show the electron
occupation of the orbitals.
.
Draw sketches of each of the orbitals and energy order them. Show the electron
occupation of the orbitals.