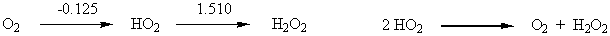Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Practice Problems
Oxidation & Reduction
1. Some anaerobic bacteria utilize oxidizing agents other than O2 as an energy source; for example, SO42–, NO3–, and Fe3+. One half-reaction is FeO(OH)(s) + HCO3–(aq) + 2 H+(aq) + e– → FeCO3(s) + 2 H2O(l), for which Eo = +1.67 V. What mass of iron gives the same standard reaction Gibbs energy as 1.00 g of oxygen?
2. Using the following aqueous acid solution reduction potentials E° (Pd2+, Pd) = +0.915 V and E° ([PdCl4]2–, Pd) = +0.600 V, calculate the equilibrium constant for Pd2+(aq) + 4 Cl–(aq) →← [PdCl4]2–(aq) in 1 M HCl(aq).
3. Many of the tabulated data for standard potentials have been determined from thermochemical data rather than direct electrochemical measurements of cell potentials. Carry out a calculation to illustrate this approach for the half-reaction Sc2O3(s) + 3 H2O(l) + 6 e– → 2 Sc(s) + 6 OH–(aq).
Sc3+(aq) OH–(aq) H2O(l) Sc2O3(s) Sc(s)
ΔHf° (kJ/mol)–614.2 –230.0 –285.8 –1908.7 0
Sm°(J K–1mol–1)–255.2 –10.75 +69.91 +77.0 +34.76
4. Calculate the reduction potential at 25 °C for the conversion of MnO4–(aq) to MnO2(s) in aqueous solution at pH = 9.00 and 1 M MnO4–(aq) given that E°(MnO4–, MnO2) = +1.69 V.
5. Balance the following redox reaction in acid solution: MnO4–(aq) + H2SO3(aq) → Mn2+(aq) + HSO4–(aq). Predict the qualitative pH dependence on the net potential for this reaction (i.e. increases, decreases, remains the same).
6. From the following Latimer diagram, calculate the net E° value for the reaction 2 HO2(aq) → O2(g) + H2O2(aq)
Given your value of E°, comment on the thermodynamic tendency of HO2 to undergo disproportionation.
7. Comment on the likelihood that the following reactions occur by a simple out-sphere electron transfer, simple atom transfer, or a multistep mechanism:
(a) HIO(aq) + I–(aq) → I2(aq) + OH–(aq)
(b) [Co(phen)3]3+(aq) + [Cr(bipy)3]2+(aq) → [Co(phen)3]2+(aq) + [Cr(bipy)3]3+(aq)
(c) IO3–(aq) + 8 I–(aq) + 6 H+(aq) → 3 I3–(aq) + 3 H2O(l)
8. Complete and balance the following oxidation-reduction reactions.
a) H2O2(aq) → O2(g) (pH = 0)
b) MnO4–(aq) + C2O42–(aq) → CO2(g) + Mn2+(aq) (pH = 0)
c) MnO4–(aq) + CH3CH2OH(aq) → CH3CHO(aq) + MnO2(s) (pH = 14)