Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Practice Problems
Molecular Structure and Covalent Bonding
1. Write Lewis structures for (a) XeF4, (b) PF5, (c) BrF3, (d) TeCl4, (e) ICl2–. Give the formal charge and oxidation number for each atom.
2. For the following species, write Lewis dot structures, predict the molecular geometry, estimate all bond angles (to ±2°), and give the hybridization of the central atom: a) ClO2–; b) SiF62–; c) CrO42–; d) XeF2.
3. Write the Lewis dot structure for the following species: a) chlorine dioxide; b) perchlorate ion; c) permanganate ion. Give the formal charge and the oxidation number for each atom in each species.
4. Predict the bond angles for a) chlorine dioxide; b) perchlorate ion; c) permanganate ion.
5. Predict the structure, including estimates of all bond angles, for the following: a) ClO3– b) XeO3; c) CrO2F2. Also give the hybridization for the central atom.
6. What shapes would you expect for the species (a) SO3, (b) SO32–, (c) IF5? Give an estimate of the bond angles (accurate to ±2o).
7. For the following species, write Lewis dot structures, predict the molecular geometry, estimate all bond angles (to ±2°), and give the hybridization of the central atom: a) ClO2–; b) SF6; c) MnO4–; d) XeOF2.
8. Label the following orbitals as σ, π, δ, σ*, π*, or δ*.
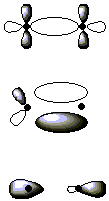
9. What are the expected changes in bond order and bond distance that accompany the following ionization process?
(a) O2 → O2+ + e–, (b) N2 + e– → N2–, (c) NO → NO+ + e–
10. Use the concepts of penetration and shielding on the radial wavefunction, to account for the variation of single bond covalent radii with position on the periodic table.
11. Use covalent radii (cite your source)) to calculate the bond lengths in (a) CCl4 (177 pm), (b) SiCl4 (201 pm), (c) GeCl4 (210 pm). (The values in parentheses are experimental bond lengths and are included for comparison.)
12. When an He atom absorbs a photon to form the excited configuration 1s12s1 (here called He*) a weak bond forms with another He atom to give the diatomic molecule HeHe*. Construct a molecular orbital description of the bonding in this species.
13. Use molecular orbital diagrams to determine the number of unpaired electrons in (a) O2–, (b) O2+, (c) BN, and (d) NO–. Also give the bond order for each species in the question.
14. Determine the MO bond orders of (a) S2, (b) Cl2, and (c) NO– from their molecular orbital configurations and compare the values with the bond orders determined from Lewis structures. (NO has orbitals like O2.)
15. The gas phase ionization potentials for some of the first row diatomic molecules are given below:
MoleculeIonization Potential (eV)
Li25.1127
C211.4
N215.5808
O212.0697
F215.897
Explain the trends in the ionization potentials using the molecular orbital diagrams presented in class.
16. Draw a molecular orbital diagram for the hypochlorite ion. What is the bond order? Is this consistent with the bonding predicted by a Lewis dot structure?
17. Draw a molecular orbital diagram for the hydroxide ion. What is the bond order? Is this consistent with the bonding predicted by a Lewis dot structure?
