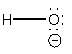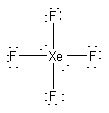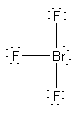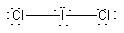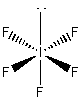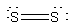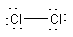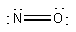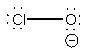Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Practice Problems
Molecular Structure and Covalent Bonding
1. Write Lewis structures for (a) XeF4, (b) PF5, (c) BrF3, (d) TeCl4, (e) ICl2–. Give the formal charge and oxidation number for each atom.
a) XeF4
Formal charges: Xe, 0; F, 0.
Oxidation numbers: Xe, +4; F, –1.
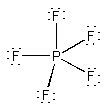
(b) PF5
Formal charges: P, 0; F, 0.
Oxidation numbers: P, +5; F, –1.
(c) BrF3
Formal charges: Br, 0; F, 0.
Oxidation numbers: Br, +3; F, –1.
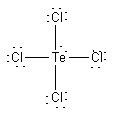
(d) TeCl4
Formal charges: Te, 0; Cl, 0.
Oxidation numbers: Te, +4; Cl, –1.
(e) ICl2–
Formal charges: I, –1; Cl, 0.
Oxidation numbers: I, +1; Cl, –1.
2. For the following species, write Lewis dot structures, predict the molecular geometry, estimate all bond angles (to ±2°), and give the hybridization of the central atom: a) ClO2–; b) SiF62–; c) CrO42–; d) XeF2.
SpeciesLewis StructureElectron Distribution GeometryBond AnglesHybrid
ClO2–
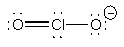
AngularO-Cl-O ~ 107°sp3
SiF62–
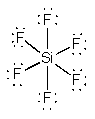
OctahedralF-Si-F = 90°d2sp3
CrO42–
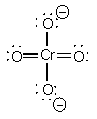
TetrahedralO-Cr-O - 109°sd3
XeF2
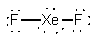
LinearF-Xe-F = 180°dsp3
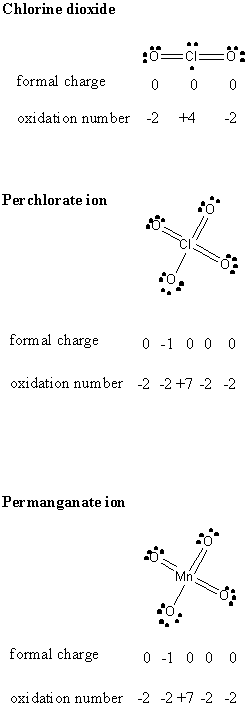
3. Write the Lewis dot structure for the following species: a) chlorine dioxide; b) perchlorate ion; c) permanganate ion. Give the formal charge and the oxidation number for each atom in each species.
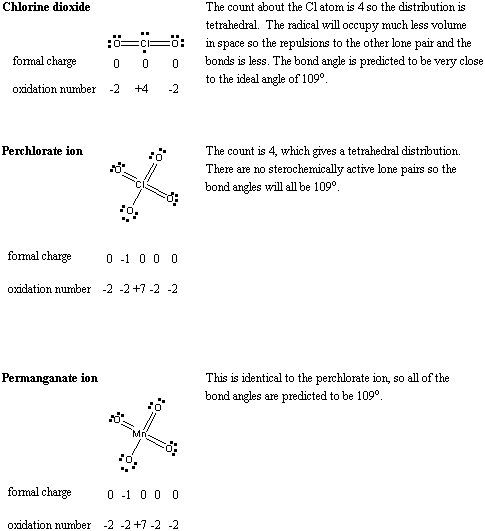
4. Predict the bond angles for a) chlorine dioxide; b) perchlorate ion; c) permanganate ion.
5. Predict the structure, including estimates of all bond angles, for the following: a) ClO3– b) XeO3; c) CrO2F2. Also give the hybridization for the central atom.
6. What shapes would you expect for the species (a) SO3, (b) SO32–, (c) IF5? Give an estimate of the bond angles (accurate to ±2o).
(a) SO3
Around the sulfur center there are 3 bonds and no lone pairs, leading to a trigonal planar distribution of electron density. Since there are no lone pairs, this is also the molecular shape. The bond angles are all 120o.
(b) SO32–
Around the sulfur center there are 3 bonds and one lone pair, leading to a tetrahedral distribution of electron density. With one lone pair, the molecular shape becomes pyramidal. The bond angles are all about 107o.
(c) IF5
Around the iodine center there are 5 bonds and one lone pair, leading to an octahedral distribution of electron density. With one lone pair, the molecular shape becomes a square-based pyramid. All of the bond angles become slightly less than 90o, perhaps about 89o.
The angles are exaggerated in the above picture.
7. For the following species, write Lewis dot structures, predict the molecular geometry, estimate all bond angles (to ±2°), and give the hybridization of the central atom: a) ClO3–; b) SF6; c) MnO4–; d) XeOF2.
SpeciesLewis StructureElectron Distribution GeometryBond AnglesHybrid
ClO3–
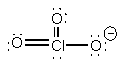
PyramidalO-Cl-O ~ 107°sp3
SF62–
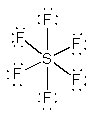
OctahedralF-S-F = 90°d2sp3
MnO4–
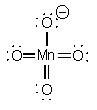
TetrahedralO-Mn-O - 109°sd3
XeOF2
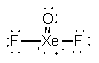
Bent - TF-Xe-F ~179°
F-Xe-O ~89°dsp3
8. Label the following orbitals as σ, π, δ, σ*, π*, or δ*.
9. What are the expected changes in bond order and bond distance that accompany the following ionization process?
(a) O2 → O2+ + e–, (b) N2 + e– → N2–, (c) NO → NO+ + e–
(a) O2 → O2+ + e–:
O2 has a bond order of 2 and O2+ has a bond order of 2.5 (the electron has been removed from a π* orbital), so the bond order increases and the bond distance decreases.
(b) N2 + e– → N2–:
N2 has a bond order of 3 and N2– has a bond order of 2.5 (the electron is put into a π* orbital) so the bond order decreases and the bond distance increases.
(c) NO → NO+ + e–:
NO has a bond order of 2.5 and NO+ has a bond order of 3 (the electron is removed from a π* orbital) so the bond order increases and the bond distance decreases.
10. Use the concepts of penetration and shielding on the radial wavefunction, to account for the variation of single bond covalent radii with position on the periodic table.
From Table 2.7 from the textbook (single bond radii only, in pm):
H 37
C 77N 74O 66F 64
Si 118P 110S 104Cl 99
Ge 122As 121Se 117Br 114
Sb 141Te 137I 133
In general, moving across the periodic table to the right decreases the covalent radius because of the increase in Z* (causing the electron density to be more strongly attracted to the nucleus).
In general, moving down the periodic table increases the covalent radius because of the increasing principal quantum number and associated size increase of the atomic orbital.
11. Use covalent radii (cite your source)) to calculate the bond lengths in (a) CCl4 (177 pm), (b) SiCl4 (201 pm), (c) GeCl4 (210 pm). (The values in parentheses are experimental bond lengths and are included for comparison.)
Covalent radii are from Table 2.7 from Shriver, Weller, et al, Inorganic Chemistry, 6th edition.
(a) CCl4 has all single bonds, so the estimated bond length is 77 + 99 = 176 pm. The calculated value agrees with experiment very well.
(b) SiCl4 has all single bonds, so the estimated bond length is 118 + 99 = 217 pm. The calculated value agrees with experiment only moderately well.
(c) GeCl4 has all single bonds, so the estimated bond length is 122 + 99 = 221 pm. The calculated value agrees with experiment only moderately well.
12. When an He atom absorbs a photon to form the excited configuration 1s12s1 (here called He*) a weak bond forms with another He atom to give the diatomic molecule HeHe*. Construct a molecular orbital description of the bonding in this species.
Use the MO diagram shown below. The 1s orbital in He* drops in energy relative to the 1s orbital in He because the electron in the 2s orbital of He* provides little shielding to the 1s electron in He*. Thus, Z* for the 1s electron in He* is close to 2, stabilizing the 1s orbital. Overlap is best between orbitals of about the same energy, so the primary overlap is between the 1s orbital on He and the 2s orbital on He*.
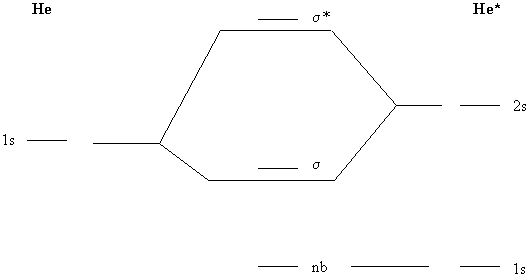
The electron configuration becomes nb2σ2 with a bond order of 1. The bond is weak because the overlap between the 1s and 2s orbitals is still small so that the stabilization due to bond is also relatively small.
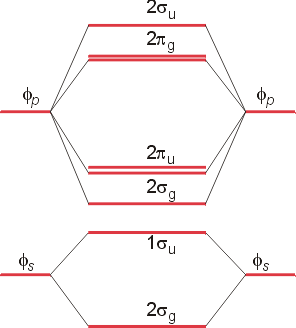
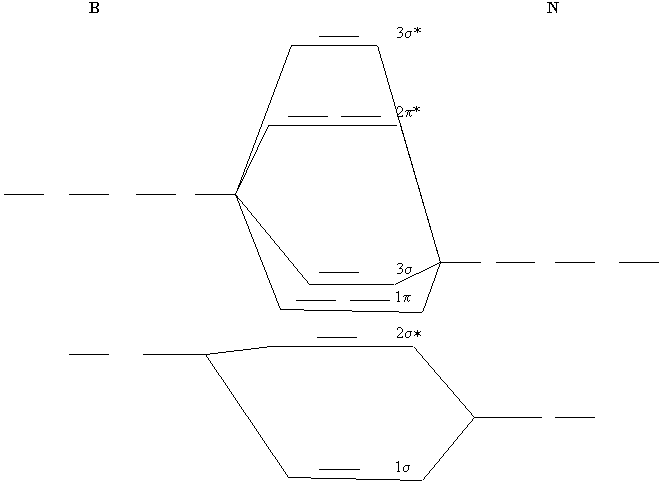
13. Use molecular orbital diagrams to determine the number of unpaired electrons in (a) O2–, (b) O2+, (c) BN, and (d) NO–. Also give the bond order for each species in the question.
(a) Use the MO diagram from Figure 2.18 from the textbook:
The electron configuration for O2– is 1σg21σu22σg22πu42πg3. This leaves 1 unpaired electron and gives a bond order of 1.5.
(b) Use the same MO diagram as in (a), giving an electron configuration for O2+ of:
1σg21σu22σg22πu42πg1. This leaves 1 unpaired electron and gives a bond order of 2.5.
(c) The MO diagram for BN can be constructed, similar to that of the homonuclear diatomics:
The electron configuration becomes 1σ22σ*21π4, the number of unpaired electrons is 0, and the bond order is 2. Other MO diagram constructions can give different electron configurations and bond orders, but should always give no unpaired spins.
(d) Use the MO diagram in part (c), changing N for B and O for N. Then, the electron configuration for NO– is:
1σ22σ*21π43σ22π*2, there are 2 unpaired electrons, and the bond order is 2.
14. Determine the MO bond orders of (a) S2, (b) Cl2, and (c) NO– from their molecular orbital configurations and compare the values with the bond orders determined from Lewis structures. (NO has orbitals like O2.)
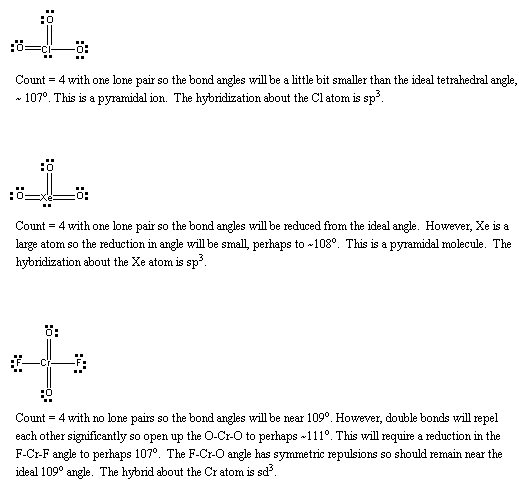
(a) S2 will be like O2, except that the basis functions are 3s and 3p rather than 2s and 2p. Thus, the molecular orbital configuration is predicted to be 1σ21σ*22σ21π41π*2 with a bond order of 2. The Lewis structure is:
This also predicts a double bond.
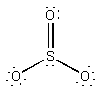
(b) Cl2 will be like F2, except that the basis functions are 3s and 3p rather than 2s and 2p. Thus, the molecular orbital configuration is predicted to be 1σ21σ*22σ21π41π*4 with a bond order of 1. The Lewis structure is:
This also predicts a single bond.
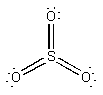
(c) NO– will be like O2. Thus, the molecular orbital configuration is predicted to be 1σ21σ*22σ21π41π*2 with a bond order of 2. The Lewis structure is:
This also predicts a double bond.
15. The gas phase ionization potentials for some of the first row diatomic molecules are given below:
MoleculeIonization Potential (eV)
Li25.1127
C211.4
N215.5808
O212.0697
F215.897
Explain the trends in the ionization potentials using the molecular orbital diagrams presented in class.
The trend from Li2 to C2 to N2 has increasing IP because the highest occupied molecular orbital (HOMO) is becoming lower in energy, a consequence of increasing Z* for the atoms. The IP for O2 drops dramatically because the HOMO is now a π* orbital of higher energy, thereby making it easier to remove the electron. The IP for F2 increases because the same π* orbital is being filled as for O2 but with lower energy because of the increase in Z* for F. The MO diagram shown below (from lecture) shows graphical details.
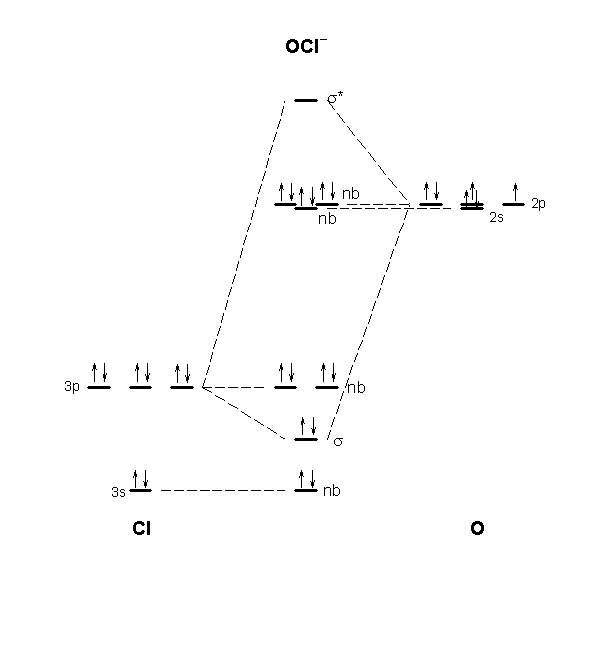
16. Draw a molecular orbital diagram for the hypochlorite ion. What is the bond order? Is this consistent with the bonding predicted by a Lewis dot structure?
The molecular orbital diagram for ClO– is given below:
The basis orbitals for Cl are 3s and 3p and for O are 2s and 2p. Z* for O 2s and 2p orbitals are similar so the AOs start at nearly the same energy. For the Cl 3s and 3p orbitals the two Z* values are quite different so the initial energies are more separated. The only significant overlap will be between the p orbitals pointing at each other to give sigma bonding and antibonding orbitals. Owing to the sizable energy and size mismatch between the 2p and 3p orbitals, there should be only insignificant pi-type interactions.
The bond order is 1, which agrees with the single bond found from the Lewis dot structure:
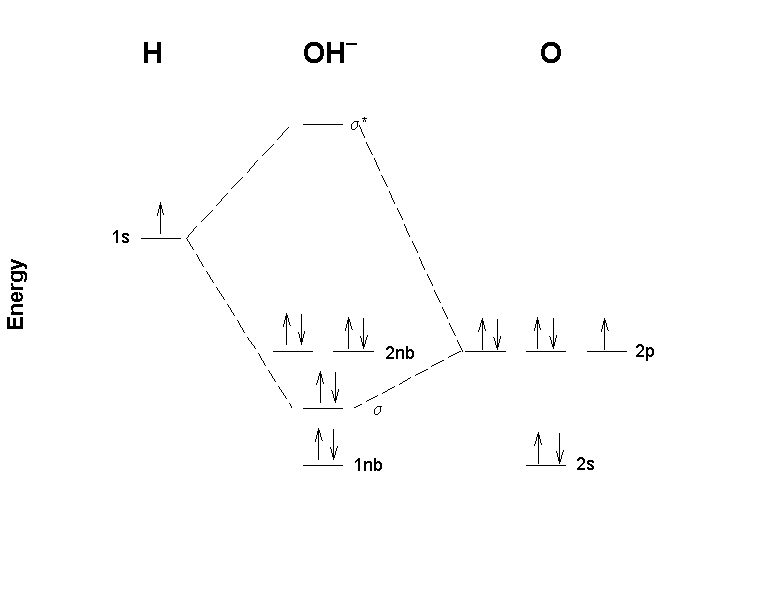
17. Draw a molecular orbital diagram for the hydroxide ion. What is the bond order? Is this consistent with the bonding predicted by a Lewis dot structure?
The basis orbital for H is 1s and for O are 2s and 2p. Z* for O 2s and 2p orbitals are similar so the AOs start at nearly the same energy.
The bond order is 1, which agrees with the single bond found from the Lewis dot structure: