Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2019
Exam 3
1. Write the balanced half-reaction and find the standard reduction potential for the reduction of dimanganese trioxide to manganese metal in base.
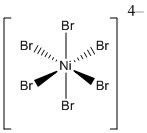
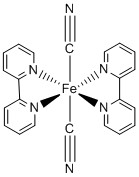
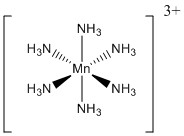
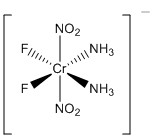
2. For the following metal complexes, give the systematic name, give the point group, find the LFSE in units of Dq and P, indicate if the complex is expected to be Jahn-Teller active, and give the spin-only magnetic moment in units of Bohr-magnetons.
a.
b.
c.
d.
3. For the following metal complexes, show the structure, give the point group, and indicate if the complex is stable by the EAN rule.
a. Hexacarbonylchromium(0)
b. Bis-(η5-cyclopentadienyl)cobalt(II)
c. Bis-(η1-cyclopentadienyl)-bis-(η5-cyclopentadienyl)titanium(IV)
d. trans-Carbonylchlorobis(triphenylphosphine)iridium(I)
Possibly useful information
Spectrochemical Series
I– < Br– < S2– < SCN– < Cl– < NO3– < F– < ox2– < H2O < SCN– < CH3CN < NH3 < en < bpy < phen < NO2– < PPh3 < CN– < CO
ox2–: –O2CCO2–
en: H2NCH2CH2NH2 bpy: 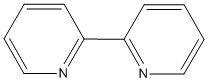 phen:
phen: 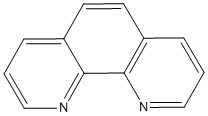 PPh3:
PPh3: 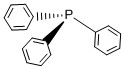
μ = [n(n+2)]½ μB
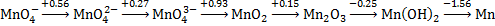 pH = 14
pH = 14