Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2019
Exam 2
1. Write the balanced half-reaction and find the standard reduction potential for the reduction of dimanganese trioxide to manganese metal in base.
Balance Mn mass: Mn2O3(s) → 2 Mn(s)
Balance O mass with H2O: Mn2O3(s) → 2 Mn(s) + 3 H2O(l)
Balance H mass with H+: Mn2O3(s) + 6 H+(aq) → 2 Mn(s) + 3 H2O(l)
Balance charge with e–: Mn2O3(s) + 6 H+(aq) + 6 e– → 2 Mn(s) + 3 H2O(l)
Neutralize H+: 6 H2O(l) → 6 H+(aq) + 6 OH–(aq)
Net half-reaction: Mn2O3(s) + 3 H2O(l) + 6 e– → 2 Mn(s) + 6 OH–(aq)
Standard reduction potential: E° = [2(–0.25) + 4(–1.56)]/[2 + 4] = –1.12 V
2. For the following metal complexes, give the systematic name, give the point group, find the LFSE in units of Dq and P, indicate if the complex is expected to be Jahn-Teller active, and give the spin-only magnetic moment in units of Bohr-magnetons.
a.
b.
c.
d.
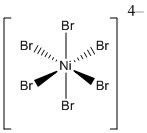
a.
Name: hexabromonickelate(II) ion
Point Group: Oh
LFSE: Ni2+ in a weak field is d8, t2g6eg2 so LFSE = 12Dq
Jahn-Teller: No
Magnetic moment: Ni2+ in a weak field has 2 unpaired spins so μ = [2(2+2)]½ = 2.83 μB
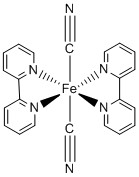
b.
Name: trans-bis(2,2'-bipyridine)dicyanoiron(II)
Point Group: D2h
LFSE: Fe2+ in a strong field is d6, t2g6 so LFSE = 24Dq – 2P
Jahn-Teller: No
Magnetic moment: Fe2+ in a strong field has 0 unpaired spins so μ = 0 μB
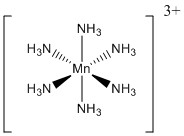
c.
Name: hexaamminemanganese(III) ion
Point Group: Oh
LFSE: Mn3+ in a strong field is d4, t2g4 so LFSE = 16Dq – P
Jahn-Teller: Yes (axial compression)
Magnetic moment: Mn3+ in a strong field has 2 unpaired spins so μ = [2(2+2)]½ = 2.83 μB
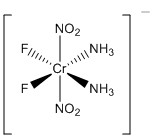
d.
Name: cis-biammine-cis-bifluoro-trans-binitritochromate(III) ion
Point Group: C2v
LFSE: Cr3+ in any field strength is d3, t2g3 so LFSE = 12Dq
Jahn-Teller: No
Magnetic moment: Cr3+ has 3 unpaired spins so μ = [3(3+2)]½ = 3.87 μB
3. For the following metal complexes, show the structure, give the point group, and indicate if the complex is stable by the EAN rule.
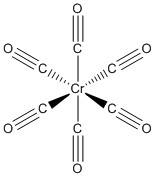
a. Hexacarbonylchromium(0)
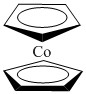
b. Bis-(η5-cyclopentadienyl)cobalt(II)
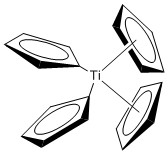
c. Bis-(η1-cyclopentadienyl)-bis-(η5-cyclopentadienyl)titanium(IV)
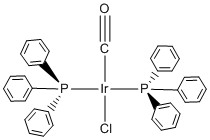
d. trans-Carbonylchlorobis(triphenylphosphine)iridium(I)
a. Hexacarbonylchromium(0)
Structure:
Point Group: Oh
EAN: Cr(0) is d6, each CO ligand contribute 2 e– so the total is 6 + 6(2) = 18 e–, stable.
b. Bis-(η5-cyclopentadienyl)cobalt(II)
Structure:
Point Group: D5d
EAN: Co(II) is d7, each cp ligand contribute 6 e– so the total is 7 + 2(6) = 19 e–, not stable.
c. Bis-(η1-cyclopentadienyl)-bis-(η5-cyclopentadienyl)titanium(IV)
Structure:
Point Group: C2v
EAN: Ti(IV) is d0, each η1 cp ligand contribute 2 e–, each η5 cp ligand contributes 6 e– so the total is 0 + 2(2) + 2(6) = 16 e–, probably stable.
d. trans-Carbonylchlorobis(triphenylphosphine)iridium(I)
Structure:
Point Group: C2v
EAN: Ir(I) is d8, the CO ligand contributes 2 e–, the Cl ligand contributes 2 e–, and each PPh3 ligand contributes 2 e– so the total is 8 + 1(2) + 1(2) + 2(2) = 16 e–, probably stable.