Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2018
Exam 1
1. Draw the Lewis structure, give the formal charge and oxidation number on each atom, predict the molecular structure, estimate all bond angles, and give the hybrid orbitals needed for the central atom(s)) for the following:
a. SOF3–
b. HPF3+
c. S52– (the S atoms are bonded in a linear fashion)
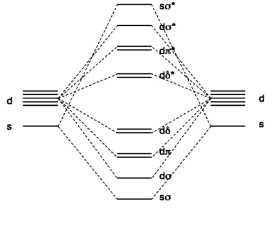
2. The molecular diagram for CrW (gas phase) is shown below (taken from F. Ruipérez, et al, Inorg. Chem. 2011, 50, 9219). What basis orbitals were used to construct the MO diagram? What is the electron configuration for each atom in the molecule? What is the bond order of the molecule?
3. Give the electron configuration and ground state term symbol for La3+, Eu3+, and Dy3+.
4. A plot of Z* for the 4s and 3d electrons for the first row transition metals is shown below. The magnitude for 4s electrons is less than for 3d electrons. Why? For both types of electrons the change across the periodic table is linear but the slopes are different. Explain this observation.