Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2018
Exam 1
1. Draw the Lewis structure, give the formal charge and oxidation number on each atom, predict the molecular structure, estimate all bond angles, and give the hybrid orbitals needed for the central atom(s)) for the following:
a. SOF3–
b. HPF3+
c. S52– (the S atoms are bonded in a linear fashion)
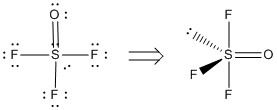
a) SOF3–
Molecular structure: see-saw or teeter-totter
Formal charges for the structure shown: S, –1; F (all 3), 0; O, 0. It is also possible to write a reasonable Lewis structure that has these formal charges: S, 0; F (all 3), 0; O, –1.
Oxidation numbers: S, +4; F (all 3), –1; O, –2
F-S-F angles: ~178° and ~88°
F-S-O angles: ~118° and ~88°
Hybrid: dsp3
b) HPF3+
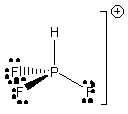
Molecular structure: tetrahedral
Formal charges: P, +1; F (all 3), 0; H, 0.
Oxidation numbers: P: +5; F (all 3), –1; H, –1.
F-P-F angles: ~111°
F-P-H angles: ~107°
Hybrid: sp3
c) S52–
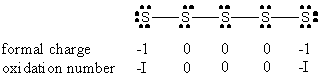
Molecular structure: W-shaped
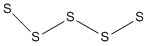
Formal charges and oxidation are shown under the Lewis structure.
All S-S-S angles are ~107°
Hybrid on all of the S atoms: sp3
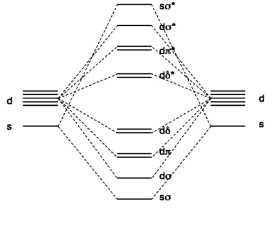
2. The molecular diagram for CrW (gas phase) is shown below (taken from F. Ruipérez, et al, Inorg. Chem. 2011, 50, 9219). What basis orbitals were used to construct the MO diagram? What is the electron configuration for each atom in the molecule? What is the bond order of the molecule?
The MO diagram shows s and d orbitals used to construct the molecular orbitals, so the basis set must be 4s and 3d (from Cr) and 6s and 5d (from W).
The atomic electron configurations for the atoms are predicted to be: Cr, [Ar]4s13d5 and W, [Xe]4f146s15d5 (the actual configuration is [Xe]4f146s25d4)
Cr and W each have 6 valence electrons, so a total of 12 electrons must be added to the molecular orbitals. This makes the molecule electron configuration: sσ2dσ2dπ4dδ4.
All 12 electrons are in bonding orbitals so the calculated bond order is 6 (this seems unrealistically high).
3. Give the electron configuration and ground state term symbol for La3+, Eu3+, and Dy3+.
La3+
La3+ is [Xe]6s05d04f0 so the term symbol is 1S
Eu3+
Eu3+ is [Xe]6s05d04f6 so L = +3 + +2 + +1 + 0 + –1 + –2 = 3 and S = ½ + ½ + ½ + ½ + ½ + ½ = 3 so the term symbol is 7F
Dy3+
Dy3+ is [Xe]6s05d04f9 so L = +3 + +3 + +2 + +2 + +1 + 0 + –1 + –2 + –3 = 5 and S = ½ + ½ + ½ + ½ + ½ = 5/2 so the term symbol is 6H
4. A plot of Z* for the 4s and 3d electrons for the first row transition metals is shown below. The magnitude for 4s electrons is less than for 3d electrons. Why? For both types of electrons the change across the periodic table is linear but the slopes are different. Explain this observation.
The magnitude of Z* for 4s electrons is lower because the 3d electrons are physically closer to the nucleus (smaller n quantum number), which means that the 3d electrons provide screening of the nuclear charge from the outermost electrons. In contrast, the 4s electrons provide little screening for the 3d electrons so that Z* is larger.
The slopes are different for similar reasons. The slope for the 4s electrons is based on one 3d electron being added for each element, which partially screens the nuclear charge. Z* for the 3d electrons changes more significantly because the each 3d electron added is being screened by other 3d electrons, which are binodal and poor screeners when at the same distance from the nucleus.