Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2017
Exam 1
1. Predict the electron configuration and give the ground state term symbol for the following: a) Cr; b) Cr+; c) Cr2+; d) Cr3+; e) Cr4+. What is the trend in the ionization energies for this series? Explain your reasoning.
2. Draw the lowest energy Lewis dot structure for each of the following, give the formal charge and the oxidation number for each atom, indicate the molecular geometry, and give the hybrid orbital for the central atom: a) SbF3; b) AlCl63–; c) ArF2; d) BrO4–.
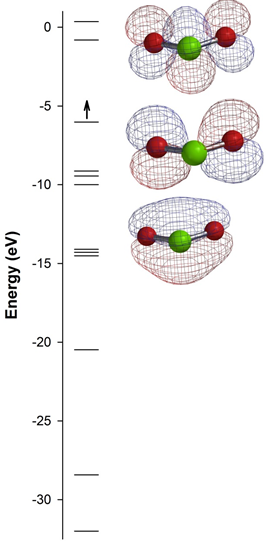
3. A molecular orbital diagram calculated for ClO2 is shown below. Based on this diagram, answer the following questions: a) what were the basis orbitals used to construct the MO diagram? b) label the three orbitals shown as σ, π, δ, σ*, π*, or δ*; c) the calculated bond angle is 119.01° – how does this compare to what you would predict using VESPR? Explain; d) the calculated bond order for each Cl-O bond is 1.26 – how can this be explained in terms of Lewis structures? e) ClO2 is an excellent disinfectant – what chemistry might drive this reactivity? The Cl atom is green, the O atoms are red, and the red and blue meshes denote the different phases of the orbitals shown.