Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2017
Exam 1
1. Predict the electron configuration and give the ground state term symbol for the following: a) Cr; b) Cr+; c) Cr2+; d) Cr3+; e) Cr4+. What is the trend in the ionization energies for this series? Explain your reasoning.
a) Cr
electron configuration: [Ar]4s13d5
Term symbol: L = (0) + (2) + (1) + (0) + (–1) + (–2) = 0; S = (½) + (½) + (½) + (½) + (½) + (½) = 3; term = 7S
b) Cr+
electron configuration: [Ar]3d5
Term symbol: L = (2) + (1) + (0) + (–1) + (–2) = 0; S = (½) + (½) + (½) + (½) + (½) = 5/2; term = 6S
c) Cr2+
electron configuration: [Ar]3d4
Term symbol: L = (2) + (1) + (0) + (–1) = 2; S = (½) + (½) + (½) + (½) = 2; term = 5D
d) Cr3+
electron configuration: [Ar]3d3
Term symbol: L = (2) + (1) + (0) = 3; S = (½) + (½) + (½) = 3/2; term = 4F
b) Cr4+
electron configuration: [Ar]3d2
Term symbol: L = (2) + (1) = 3; S = (½) + (½) = 1; term = 3F
The trend in ionization potential is that the IP increases as the charge on the ion increases. This is due to the increasing Coulombic attraction from the ion and the exiting electron.
2. Draw the lowest energy Lewis dot structure for each of the following, give the formal charge and the oxidation number for each atom, indicate the molecular geometry, and give the hybrid orbital for the central atom: a) SbF3; b) AlCl63–; c) ArF2; d) BrO4–.
a) SbF3
Lewis structure:
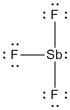
formal charges: Sb, 0; all three F, 0
oxidation numbers: Sb, +3; all three F, –1
molecular structure:
, pyramidal
hybrid orbital: sp3
b) AlCl63–
Lewis structure:
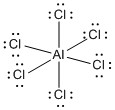
formal charges: Al, –3; all six Cl, 0
oxidation numbers: Al, +3; all six Cl, –1
molecular structure:
, octahedral
hybrid orbital: d2sp3
c) ArF2
Lewis structure:
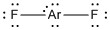
formal charges: Ar, 0; both F, 0
oxidation numbers: Ar, +2; both F, –1
molecular structure: linear
hybrid orbital: dsp3
d) BrO4–
Lewis structure:
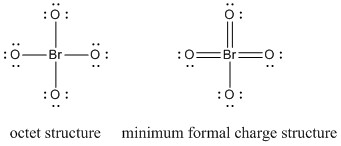
formal charges: for the octet structure, Br, +3; all four O, –1; for the minimum formal charge structure, Br, 0; three O, 0, one O, –1 so the average formal charge on O is –1/4
oxidation numbers: Br, +7; all four O, –2
molecular structure:
, tetrahedral
hybrid orbital: sp3
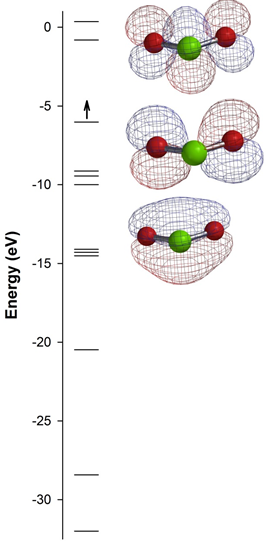
3. A molecular orbital diagram calculated for ClO2 is shown below. Based on this diagram, answer the following questions: a) what were the basis orbitals used to construct the MO diagram? b) label the three orbitals shown as σ, π, δ, σ*, π*, or δ*; c) the calculated bond angle is 119.01° – how does this compare to what you would predict using VESPR? Explain; d) the calculated bond order for each Cl-O bond is 1.26 – how can this be explained in terms of Lewis structures? e) ClO2 is an excellent disinfectant – what chemistry might drive this reactivity? The Cl atom is green, the O atoms are red, and the red and blue meshes denote the different phases of the orbitals shown.
a) There are 12 MOs shown, which means that there must be 12 basis orbitals. The most likely basis set are the 2s and 2p orbitals on the O atoms and the 3s and 3p orbitals on the Cl atom.
b) The top orbital has a planar node containing the Cl-O bonds, which indicates a π type orbital, and planar nodes that are perpendicular to each each Cl-O bond and between the bonds, which indicates an antibonding orbital, thus the MO is a π*.
The middle orbital has a planar node containing the Cl-O bonds, which indicates a π type orbital, but no node perpendicular to the Cl-O bonds between atom (there is a perpendicular node that contains the Cl atom), thus the MO is π.
The bottom orbital has a planar node containing the Cl-O bonds, which indicates a π type orbital, but no node perpendicular to the Cl-O bonds between atom, thus the MO is π.
c) Two possible low energy Lewis structures (resonance structure) are
. Either structure implies a planar bent structure with a O-Cl-O bond angle of about 107-108° if the single electron acts similarly to a lone pair. This prediction completely disagrees with the calculated angle of 119°. If the lone electron is ignored, then a bond angle of ~119° is predicted, but this seems unreasonable.
d) A bond order of 1.26 suggests that the best VBT prediction is about 75% contribution from the Lewis structure with single bonds (left above) and about 25% of the double bond structure (right above).
e) There is a single electron in the highest occupied orbital that could be easily lost via oxidation (to form ClO2+) or paired up via reduction (to form ClO2–). ClO2 disinfects by redox chemistry.