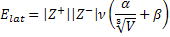Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2012
Exam 2
1. Consider the following equation used to estimate lattice energies for ionic salts, MpXq:
where ν is the number of ions per molecule (= p + q), Z+ and Z– are the ionic charges, V is the ionic volume in nm3, α = 138.7 kJ/mol, and β = 27.6 kJ/mol. The ionic volume is found from V = pV+ + qV– and V+ and V– are found from ionic radii (V = 4πr3/3). Use this formula to estimate the lattice energy for NaCl. The ionic radius of Na+ is 102 pm and the ionic radius of Cl– is 181 pm. Comment on the reliability of this method, recalling that the lattice energy found from the Born-Landé equation is 750.2 kJ/mol and the experimental lattice energy is 787.1 kJ/mol.
2. The following redox reaction can be induced by pressure: Cs2PdI4 + I2 → Cs2PdI6. Which atom is oxidized and which atom is reduced? The high-pressure phase (Cs2PdI6) is a close-packed structure (face-centered cubic); which ion establishes the lattice packing and which ion or ions fill the holes? Why?
3. Based on the calculated the electronic band structure for K1/3Ba2/3AgTe2, this material must be a semiconductor. Would you expect this material to be a p-type or n-type semiconductor? Explain why. Hint: assume that the metal ions exist in their most common oxidation states.
4. Consider the compound (C6F13)2P(O)OH. Would you expect this acid to have a pKa higher or lower than phosphoric acid? Briefly explain your reasoning.
5. Balance the following reaction:
Co(CH3CO2)3(aq) + HBr(aq) → CoBr2(aq) + CH3CO2H(aq) + Br2(l)