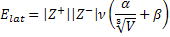Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2012
Exam 2
1. Consider the following equation used to estimate lattice energies for ionic salts, MpXq:
where ν is the number of ions per molecule (= p + q), Z+ and Z– are the ionic charges, V is the ionic volume in nm3, α = 138.7 kJ/mol, and β = 27.6 kJ/mol. The ionic volume is found from V = pV+ + qV– and V+ and V– are found from ionic radii (V = 4πr3/3). Use this formula to estimate the lattice energy for NaCl. The ionic radius of Na+ is 102 pm and the ionic radius of Cl– is 181 pm. Comment on the reliability of this method, recalling that the lattice energy found from the Born-Landé equation is 750.2 kJ/mol and the experimental lattice energy is 787.1 kJ/mol.
First, find the ionic volumes: V+ = 4πr+3/3 = 4π(0.102)3/3 = 0.00445 nm3 and V– = 4πr–3/3 = 4π(0.181)3/3 = 0.0248 nm3. Then, V = 0.00445 + 0.0248 = 0.0293 nm3. V⅓ = (0.0293)⅓ = 0.308 nm. Then, Elat = |+1||-1|(2)(138.7/0.308 + 27.6) = 955 kJ/mol.
The result is poor, overestimating the lattice energy by 21 %. The Born-Landé makes a much better estimate, but does require one additional parameter (n - the Born exponent). This method is designed to find reasonable answers for a wide variety of ionic compounds but the reliability for any given example can be less than desired.
2. The following redox reaction can be induced by pressure: Cs2PdI4 + I2 → Cs2PdI6. Which atom is oxidized and which atom is reduced? The high-pressure phase (Cs2PdI6) is a close-packed structure (face-centered cubic); which ion establishes the lattice packing and which ion or ions fill the holes? Why?
Cs+2Pd2+I–4 + I02 → Cs+2Pd4+I –6
The Pd atom is oxidized and the I atom in molecular iodine is reduced.
The I–; is large, so should be expected to form the lattice. The smaller Cs+ and Pd4+ ions fill the holes.
3. Based on the calculated the electronic band structure for K1/3Ba2/3AgTe2, this material must be a semiconductor. Would you expect this material to be a p-type or n-type semiconductor? Explain why. Hint: assume that the metal ions exist in their most common oxidation states.
K1/3Ba2/3AgTe2: the hint tells us that the metal oxidation states are K+, Ba2+, and Ag+, requiring the Te to be –[(1/3)(+1) + (2/3)(+2) + (+1)]/2 = –4/3. Since the natural oxidation state of Te is Te2–, there is an excess of positive charge on the Te atoms, implying the the compound is a p-type semiconductor.
4. Consider the compound (C6F13)2P(O)OH. Would you expect this acid to have a pKa higher or lower than phosphoric acid? Briefly explain your reasoning.
Phosphoric acid is P(O)(OH)3 with pKa1 estimated to be ~8 – 5 = 3, using Pauling's rules. Pauling's rules are based on electronegativity arguments, i.e., the X=O group is highly electronegative, thereby drawing electron density away from the rest of the molecule and making any OH bonds more acidic. The C6F13 group has a huge number of F atoms attached, thus could be expected to be even more electronegative than the oxo group. Thus, (C6F13)2P(O)OH is predicted to be a stronger acid than phosphoric acid and have a lower pKa.
5. Balance the following reaction:
Co(CH3CO2)3(aq) + HBr(aq) → CoBr2(aq) + CH3CO2H(aq) + Br2(l)
Oxidation half-reaction:
2 HBr(aq) → Br2(l) + 2 H+(aq) + 2 e–
Reduction half-reaction:
Co(CH3CO2)3(aq) + 2 HBr(aq) + H+(aq) + e– → CoBr2(aq) + 3 CH3CO2H(aq)
Net reaction:
2 Co(CH3CO2)3(aq) + 6 HBr(aq) → 2 CoBr2(aq) + 6 CH3CO2H(aq) + Br2(l)