Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2008
Exam 1
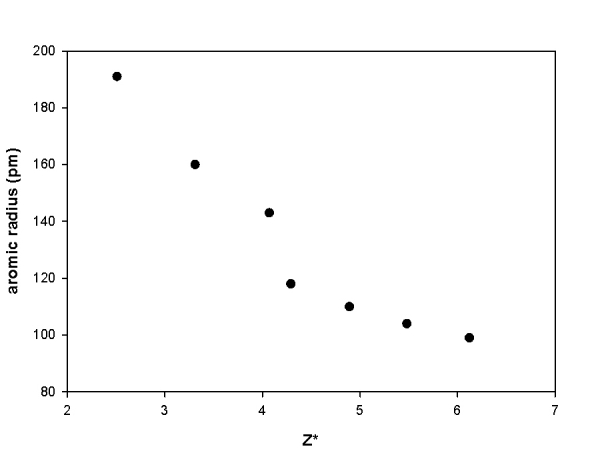
1. A plot of the atomic radius (defined as the metallic radius for metals and the covalent radius for nonmetals) vs. Z* for the elements from Na to Cl is shown below. Explain the trend in the data, including any anomalies.
2. Find the electron configuration and ground state term symbol for a) Fe3+; b) Cu+; c) Te2–; d) La3+; e) Mn2+.
3. Write the Lewis dot structure showing the formal charges, predict the structure including an estimate of all bond angles, and indicate the likely hybrid orbital on the central atom for the following: a) XeOF2; b) ICl4–; c) SO42–; d) OF2.
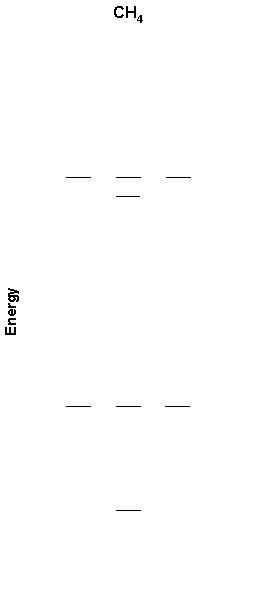
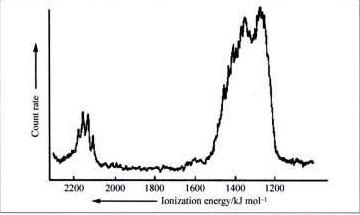
4. A partial MO diagram for methane is shown below. Label the orbitals as σ, π, δ, bonding, antibonding and find the bond order. Also shown is the photoelectron spectrum of methane. Is the photoelectron spectrum consistent with the MO diagram? Why or why not?