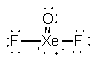Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2008
Exam 1
1. A plot of the atomic radius (defined as the metallic radius for metals and the covalent radius for nonmetals) vs. Z* for the elements from Na to Cl is shown below. Explain the trend in the data, including any anomalies.
In general, the radius decreases as Z* increases. This is as expected: as the effective nuclear charge increases the valence electrons are held more closely to the nucleus so that the atomic size is reduced. There is a step and slope change between Al and Si, which is probably attributable to the change from metallic radii (Na, Mg, Al) to covalent radii (Si, P, S, Cl).
2. Find the electron configuration and ground state term symbol for a) Fe3+; b) Cu+; c) Te2–; d) La3+; e) Mn2+.
a) Fe3+
[Ar]3d5
↑ ↑ ↑ ↑ ↑ so L = 0 and S = 5/2 giving 6S
+2 +1 0 –1 –2
b) Cu+
[Ar]3d10
Closed shell so L = 0 and S = 0 giving 1S
c) Te2–
[Xe]
Closed shell so L = 0 and S = 0 giving 1S
d) La3+
[Xe]
Closed shell so L = 0 and S = 0 giving 1S
a) Mn2+
[Ar]3d5
↑ ↑ ↑ ↑ ↑ so L = 0 and S = 5/2 giving 6S
+2 +1 0 –1 –2
3. Write the Lewis dot structure showing the formal charges, predict the structure including an estimate of all bond angles, and indicate the likely hybrid orbital on the central atom for the following: a) XeOF2; b) ICl4–; c) SO42–; d) OF2.
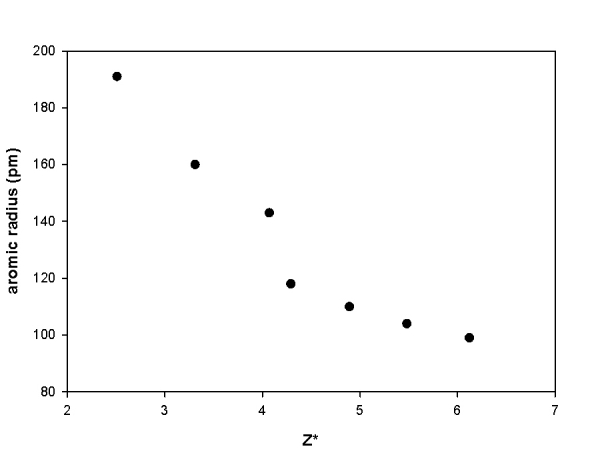
a) XeOF2
Lewis Structure:
Formal charges: Xe, 0; O, 0; F, all 0
Structure: Bent-T (O and lone pairs occupy the equatorial positions) with bond angles F-Xe-F ~189° and F-Xe-O ~89°
Hybrid orbital on Xe: dsp3
b) ICl42–
Lewis Structure:
Formal charges: I, –1; Cl, all 0
Structure: square planar with bond angle Cl-I-Cl, 180°
Hybrid orbital on I: d2sp3
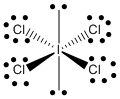
c) SO42–
Lewis Structure:
Formal charges: S, 0; O (with double bond), 0; O (with single bonds), –1
Structure: tetrahedral with bond angles: O-S-O, ~109°
Hybrid orbital on S: sp3
d) OF2
Lewis Structure:
Formal charges: O, 0; F, both 0
Structure: bent with bond angles: F-O-F, ~107°
Hybrid orbital on O: sp3
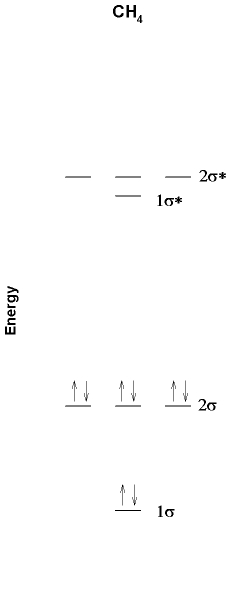
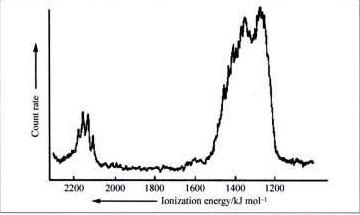
4. A partial MO diagram for methane is shown below. Label the orbitals as σ, π, δ, bonding, antibonding and find the bond order. Also shown is the photoelectron spectrum of methane. Is the photoelectron spectrum consistent with the MO diagram? Why or why not?
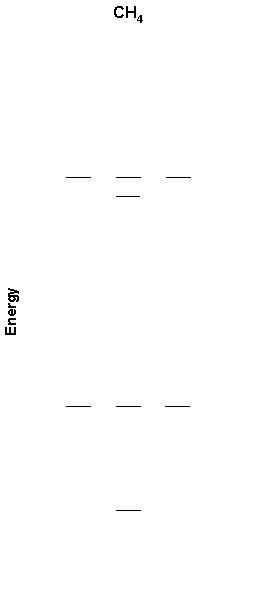
The MO diagram with labels is shown below. Using the 1s orbitals on H and the 2s and 2p orbitals on C as the basis set requires that there be 8 molecular orbitals, as shown. The lower four orbitals in the MO diagram must be σ bonding and the upper four orbitals must be σ antibonding. There are 8 electrons, which fill the bonding orbitals, so the bond order = 4.
The photoelectron spectrum has a large peak at lower energy (~1300 kJ/mole) and a small peak at higher energy (~2150 kJ/mol). This is consistent with the triply degenerate set of bonding orbitals (2σ, more intensity) in the MO diagram being higher in energy (thereby requiring less energy to ionize in the photoelectron spectrum). Likewise, the single bonding orbital (1σ) is found at higher energy in the photoelectron spectrum with lower intensity.