Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2006
Final Exam
All references are to volume 45 of Inorganic Chemistry (2006).
1. N. V. Mockus, J. L. Petersen, and J. J. Rack (pages 8–10) investigated some of the properties of [Os(bpy)2(DMSO)2]2+, where DMSO is (CH3)2S=O (dimethylsulfoxide). DMSO is an ambidentate ligand that, in this complex, bonds in a cis geometry through the S atoms. Name the complex and estimate the N-Os-N, N-Os-S, C-S-C, and C-S-O bond angles.
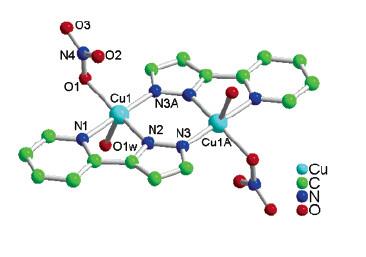
2. T.-L. Hu, J.-R. Li, C.-S. Liu, X.-S. Shi, J.-N. Zhou, X.-H. Bu, and J. Ribas (pages 162–173) studied the complex shown below. Find the point group for the complex. The measured magnetic moment at room temperature is 2.37 μB. Suggest an explanation for this value. Hint: both copper atoms are in the +2 oxidation state.
3. M. Finze, E. Bernhardt, H. Willner, and C. W. Lehman (pages 669–678) examined the reactivity of (CF3)3BCO. Predict the reaction product for the following reaction and explain your reasoning:
(CF3)3BCO + NH3 → ?
4. F. Kraus, T. Hanauer, and N. Korber (pages 1117–1123) calculated that As42– should be aromatic. Draw the Lewis dot structure for this ion and explain how aromaticity could be attained.
5. C. Chazel, M. Ménétrier, L. Croguenne, and C. Delmes (pages 1184–1191) synthesized LixNiO2, where x = 0.25. Assign the formal oxidation states of each atom in this compound. They report that the electrical conductivity is thermally activated. Does this suggest that LixNiO2 is a metal or a semiconductor? Why?
6. H. D. B. Jenkins and L. Glasser (pages 1754–1756) proposed a simple method for finding lattice energies for 1:1 salts with Z+ = +2 and Z– = –2 using only the ionic volume:
Elat = 8(119/V1/3 + 60)
where V is the ionic volume in units of nm3 and Elat is in kJ/mol. In one of the class assignments it was found that the lattice energy for ZnO (wurtzite structure) was 4008 kJ/mol using a Born-Haber cycle. Calculate the lattice energy using Jenkins & Glasser's formula and compare the result to the experimental value. Explain any discrepancies.
7. J. L. Smithback, J. B. Helms, E. Schutte, S. M. Woessner, and B. P. Sullivan (pages 2163–2174) used 2,2'-bipyridine-cis-bis(carbonyl)-trans-bis(triphenylphosphine)rhenium(I) as a reagent in a synthesis. Draw the structure of this ion, determine if it is stable by the EAN rule, and predict the magnetic moment.
8. J.-J. Wang, C. Tessier, and R. H. Holm (pages 2979–2988) discuss the reaction of selenate ion with a Mo complex, as shown below:
SeO42–(aq) + [Mo(OH)(S2C2(CH3)2)2]1–(aq) → SeO32–(aq) + [Mo(O)(OH)(S2C2(CH3)2)2]1–(aq)
where S2C2(CH3)22– (–S-C(CH3)=C(CH3)-S–) is a chelating ligand. Balance the reaction and indicate which species are oxidized and reduced. Would you expect this oxidation-reduction reaction to be inner sphere or outer sphere. Explain your reasoning.
9. Ellis (pages 3167–3186) in a review paper cites Ni(CO)4, [Co(CO)4]–, and [Fe(CO)4]2– as examples of interesting organometallic compounds. Predict the structure and stability of each of these complexes. Give the formal oxidation state of the metal in each case.
10. R. Beaulac, P. L. W. Tregenna-Piggott, A.-L. Barra, H. Weihe, D. Luneau, and C. Weber (pages 3399–3407) characterized the compound [V(urea)6](ClO4)3, where urea is H2N(C=O)NH2, which coordinates through the O atom. They find the lowest spin allowed transition in the optical spectrum to be at ~16000 cm–1. Find 10Dq (in units of cm–1) and predict the geometry around the V atom. Would you expect urea to be a weak field or strong field ligand? Explain your reasoning.