Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2000
Exam 2
1. Label each of the orbitals shown as σ, π, δ, σ*, π*, or δ*.
a) b)
b)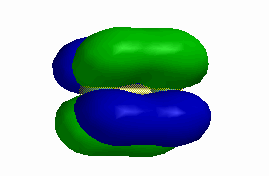
c) d)
d)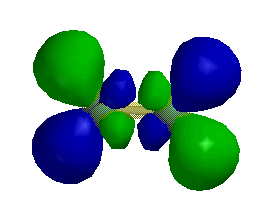
2. Construct a molecular orbital energy diagram for the CN molecule. What is the bond order? How many unpaired spins are there? Assume hybridization.
3. In Exam 1 you were asked to predict the trend in the density of the metals from Sc to Zn using mass and Z*. The nature of the bonding in these metals also is important. How does the bonding influence the density? How does the metallic bond energy vary across these series of metals?
4. Determine if the following semiconductors are n-type, p-type, or neither: a) Al doped in Si; b) S doped into Ge; c) GaAs. Briefly explain your reasoning in each case.
5. Give the point group for each of the following: a) PCl3; b) PCl5; c) [PtCl4]2–; d) O2; e) SF6.
