Chemistry 401
Intermediate Inorganic Chemistry
University of Rhode Island
Fall 2000
Exam 2
1. Label each of the orbitals shown as σ, π, δ, σ*, π*, or δ*.
a) b)
b)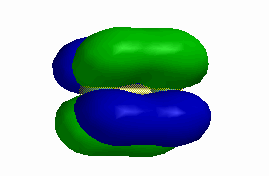
c) d)
d)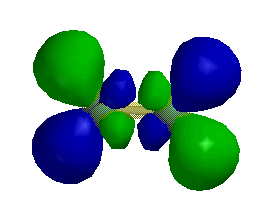
a)
0 nodes perpendicular to the internuclear axis
1 node parallel to the internuclear axis
so this is a π orbital
b)
0 nodes perpendicular to the internuclear axis
2 nodes parallel to the internuclear axis
so this is a δ orbital
c)
0 nodes perpendicular to the internuclear axis
0 nodes parallel to the internuclear axis
so this is a σ orbital
d)
1 node perpendicular to the internuclear axis
1 node parallel to the internuclear axis
so this is a π* orbital
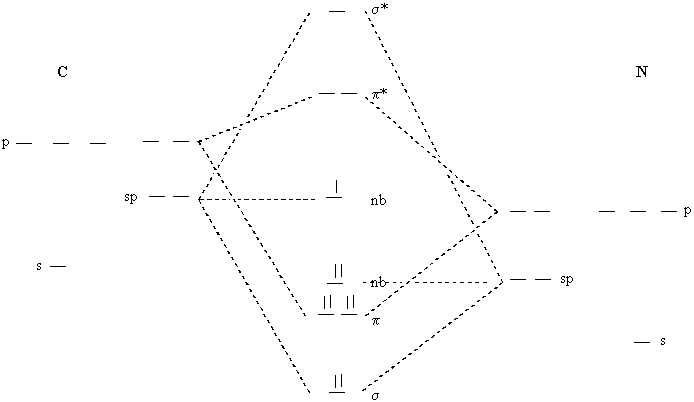
2. Construct a molecular orbital energy diagram for the CN molecule. What is the bond order? How many unpaired spins are there? Assume hybridization.
Bond order = [6 – 0]/2 = 3
1 unpaired spin
3. In Exam 1 you were asked to predict the trend in the density of the metals from Sc to Zn using mass and Z*. The nature of the bonding in these metals also is important. How does the bonding influence the density? How does the metallic bond energy vary across these series of metals?
For the transition metals the valence orbitals are 4s and 3d allowing for the possibility of 6 bands being created. Thus, net bonding should increase until 6 valence electrons have been reached (Cr) and then decrease after that since after each of the bands is half-filled antibonding states must be occupied. With more bonding character the atoms in the structure should be closer together thereby reducing the molar volume and increasing the density. Thus, density should increase sharply from Sc to Cr because all factors (mass, Z*, and bonding) are working together. After Cr the net metallic bonding is decreasing while mass and Z* are still increasing so there are competing effects. The result is that density increases more slowly from Mn to Cu and drops some for Zn. A plot is shown below.
4. Determine if the following semiconductors are n-type, p-type, or neither: a) Al doped in Si; b) S doped into Ge; c) GaAs. Briefly explain your reasoning in each case.
a) Al has fewer valence electrons than Si so will give a p-type semiconductor.
b) S has more valence electrons than Ge so will give an n-type semiconductor.
c) GaAs is a stoichiometric compound with no doping so is an intrinsic semiconductor, i.e. neither n-type nor p-type.
5. Give the point group for each of the following: a) PCl3; b) PCl5; c) [PtCl4]2–; d) O2; e) SF6.
a)
C3v
b)
D3h
c)
D4h
d)
D∞h
e)
Oh