 Exam 1B, Spring 2002
Exam 1B, Spring 2002 Exam 1B, Spring 2002
Exam 1B, Spring 20021. Bromate ion reacts with solid iodine molecules to give liquid bromine molecules and iodate ions. The rate of loss of bromate ions under some conditions is found to be 1×103 mol/L/s.
a) Under the same conditions, what is the rate of loss of iodine molecules?
b) Under the same conditions, what is the rate of gain of iodate ions?
2. Hypochlorite ion reacts with iodide ion in the presence of hydroxide to give hypoiodite ion and chloride ion. The following data was found:
[hypochlorite] (M) [iodide] (M) [hydroxide] (M) Initial Rate (mol/L/s)
1.00 1.00 1.00 60
2.00 1.00 1.00 120
1.00 2.00 1.00 120
1.00 1.00 2.00 30
a) Find the rate law.
b) Find the rate constant and give the units.
3. Rate constants were measured for some reaction at several temperatures and the following plots were made:
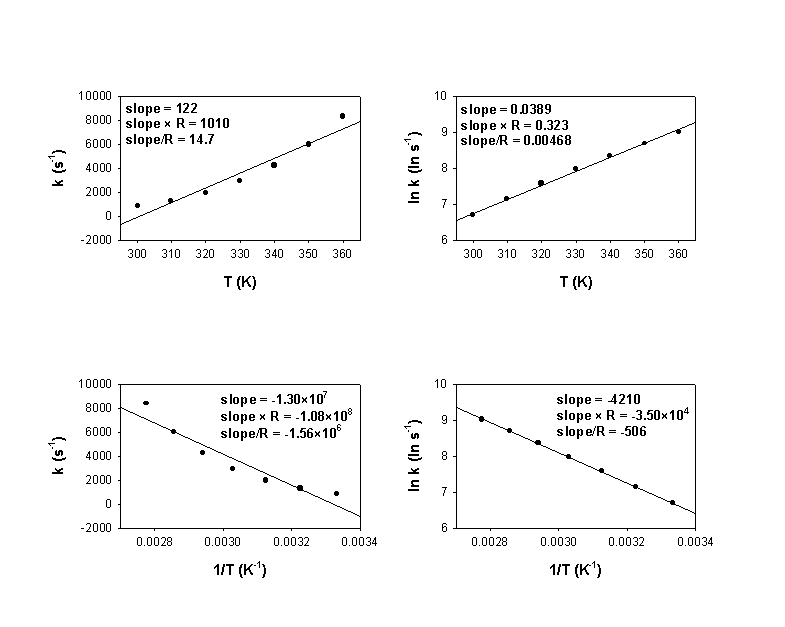
a) What is the activation energy for the reaction in units of kJ/mol?
b) What is the order of the reaction? Why?
4. Write the mass action expressions for Kp for the following reactions:
a) N2(g) + O2(g) → ← N2O4(g)
b) KClO3(s) → ← KCl(s) + O2(g)
c) Cl2(g) + Br2(g) → ← BrCl3(g)
5. The following mechanism was proposed for the reaction between hydrogen and nitrogen to give ammonia:
H2(g) + Fe(s) → FeH2(s)
FeH2(s) + N2(g) 7rarr; Fe(s) + N2H2(g)
FeH2(s) + N2H2(g) → Fe(s) + N2H4(g)
FeH2(s) + N2H4(g) → Fe(s) + 2 NH3(g)
a) The mechanism is incomplete because it does not match the reaction stoichiometry. Correct the problem and write the net reaction.
b) What is the molecularity of each reaction in the mechanism?
c) The reaction has a higher rate when iron powder is used rather than a block of iron. Why?
d) The reaction also proceeds in the absence of iron. What experimental modification would be required to maintain the rate if iron were removed from the reaction mixture?
6. The following reaction was allowed to come to equilibrium.
ClO2(g) → ← Cl2(g) + O2(g) ΔHo = –102.5 kJ/mole
Which way does the reaction shift if
a) chlorine dioxide is added to the reaction?
b) the temperature is raised?
c) the pressure is increased by reducing the volume of the reaction vessel?
7. Find the equilibrium constant Kc for the reaction:
2A(g) + B(g) → ← G(g) + H(g)
given the following information:
A(g) + B(g) → ← C(g) + D(g) Kc = 1
E(g) + F(g) → ← A(g) + C(g) Kc = ½
D(g) + E(g) → ← G(g) Kc = ½
H(g) → ← F(g) Kc = 4