 Practice Problems
Chemical Equilibrium
Practice Problems
Chemical Equilibrium Practice Problems
Chemical Equilibrium
Practice Problems
Chemical Equilibrium1. Describe how the equilibrium constant for an overall reaction is related to the equilibrium constants for the individual reactions that yield the overall reaction.
2. What role does a catalyst play in a reversible chemical reaction? How does a catalyst affect the value of the equilibrium constant for a reaction?
3. Write equilibrium constant expressions, Kp, for the following reactions.
(a) CO(g) + H2O(g) → ← CO2(g) + H2(g)
(b) N2(g) + 3 H2(g) → ← 2 NH3(g)
(c) NH4HS(s) → ← NH3(g) + H2S(g)
4. Write an equilibrium constant expression, Kp, based on the formation of one mole of each of the following gaseous compounds from its elements at 25 °C.
(a) NO(g) (b) NH3(g) (c) NOCl(g)
5. Write an equilibrium constant expressions, Kc, for each of the following reversible reactions.
(a) Carbon monoxide gas reduces nitrogen monoxide gas to gaseous nitrogen; carbon dioxide gas is the other product.
(b) Oxygen gas oxidizes gaseous ammonia to gaseous nitrogen monoxide; water vapor is the other product.
(c) Solid sodium hydrogen carbonate decomposes to form solid sodium carbonate, water vapor, and carbon dioxide gas.
6. In which of the following reactions will the amount of product at equilibrium increase if the total gas pressure is raised from 1 atm to 10 atm? Explain.
(a) SO2(g) + Cl2(g) → ← SO2Cl2(g)
(b) N2(g) + O2(g) → ← 2 NO(g)
(c) SO2(g) + ½ O2(g) → ← SO3(g)
7. Determine the values of Kc that correspond to the following values of Kp.
(a) SO2Cl2(g) → ← SO2(g) + Cl2(g)
Kp = 2.9×10–2 at 303 K
(b) 2 NO2(g) → ← 2 NO(g) + O2(g)
Kp = 0.275 at 700 K
(c) CO(g) + Cl2(g) → ← COCl2(g)
Kp = 22.5 at 395 °C
8. Determine the values of Kp that correspond to the following values of Kc.
(a) CO(g) + Cl2(g) → ← COCl2(g)
Kc = 1.2×103 at 668 K
(b) 2 NO(g) + Br2(g) → ← 2 NOBr(g)
Kc = 1.32×10–2 at 1000 K
(c) 2 COF2(g) → ← CO2(g) + CF4(g)
Kc = 2.00 at 1000 °C
9. For the reaction N2(g) + O2(g) → ← 2 NO(g), Kc = 4.08×10–4 at 2000 K.
What is the value of Kc at 2000 K for the reaction NO(g) → ← ½ N2(g) + ½ O2(g)?
10. Determine Kc at 298 K for the reaction 2 CH4(g) → ← C2H2(g) + 3 H2(g), given the following data at 298 K.
CH4(g) + H2O(g) → ← CO(g) + 3 H2(g) Kp = 1.2×10–25
2 C2H2(g) + 3 O2(g) → ← 4 CO(g) + 2 H2O(g) Kp = 1.1×102
H2(g) + ½ O2(g) → ← H2O(g) Kp = 1.1×1040
(Hint: How are Kc and Kp for the reaction related?)
11. Determine Kc at 298 K for the reaction ½ N2(g) + ½ O2(g) + ½ Cl2(g) → ← NOCl(g), given the following data set at 298 K.
½ N2(g) + O2(g) → ← NO2(g) Kp = 1.0×10–9 (1)
NOCl(g) + ½ O2(g) → ← NO2Cl(g) Kp = 1.1×102 (2)
NO2(g) + ½ Cl2(g) → ← NO2Cl(g) Kp = 0.3 (3)
(Hint: How are Kc and Kp for the reaction related?)
12. If the equilibrium concentrations found in the reaction A(g) + 2 B(g) → ← 2 C(g) are [A] = 0.025 M, [B] = 0.15 M, and [C] = 0.55 M, calculate the value of Kc.
13. In a sealed 1.75 L vessel at 250 °C, equilibrium is established between PCl5(g) and its dissociation products, PCl3(g) and Cl2(g). The quantities found at equilibrium are 0.562 g PCl5, 1.950 g PCl3, and 1.007 g Cl2. What is the value of Kc for the reaction PCl5(g) → ← PCl3(g) + Cl2(g)? What is the value of Kp?
14. Ammonium hydrogen sulfide dissociates into ammonia gas and hydrogen sulfide gas. If we start with a sample of pure NH4HS(s) at 25 °C, the total pressure of the gases is 0.658 atm when equilibrium is established. Write an equation for the dissociation reaction and determine the value of Kp
15. The equilibrium constant for the isomerization of butane at 25 °C is Kc = 7.94.
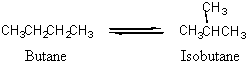
If 5.00 g butane is introduced into a 12.5 L flask at 25 °C, what mass of isobutane will be present when equilibrium is reached?
16. For the water-gas reaction {C(s + H2O(g → ← CO(g) + H2((em>g)], Kc = 0.111 at about 1100 K. If 0.100 mol H2O(g) and 0.100 mol H2(g) are mixed with excess C(s) at this temperature and equilibrium is established, how many moles of CO(g) will be present? No CO(g) is present initially.
17. For the reaction CO(g) + H2O(g) → ← CO2(g) + H2(g), Kc = 23.3 at 600 K.
If 0.250 mol each of CO and H2O are introduced into a reaction vessel and equilibrium is established, how many moles each of CO2 and H2 will be present at equilibrium? (Hint: Does the volume of the reaction mixture matter?)
18. To establish equilibrium in the following reaction at 250 °C,
PCl3(g) + Cl2(g) → ← PCl5(g) Kc = 26 at 250 °C
0.100 mol each of PCl3 and Cl2 and 0.0100 mol PCl5 are introduced into a 6.40 L reaction flask. How many moles of each of the gases will be present when equilibrium is established?
19. An analysis of the gaseous phase [S2(g) and CS2(g)] present at equilibrium at 1009 °C in the reaction
C(s) + S2(g) → ← CS2(g)
shows it to be 13.71% C and 86.29% S, by mass. What is Kc for this reaction?