 Practice Problems
Chemical Equilibrium
Practice Problems
Chemical Equilibrium Practice Problems
Chemical Equilibrium
Practice Problems
Chemical Equilibrium1. Describe how the equilibrium constant for an overall reaction is related to the equilibrium constants for the individual reactions that yield the overall reaction.
The Law of Multiple Equilibria states that when reactions are added, the corresponding equilibrium constants are multiplied. Thus, individual reactions are added to give the net reaction and the equilibrium constants of the individual reactions are multiplied to give the equilibrium constant for the net reaction.
2. What role does a catalyst play in a reversible chemical reaction? How does a catalyst affect the value of the equilibrium constant for a reaction?
Catalysts increase the rate of a chemical reaction but do not affect the final composition of a reaction. Thus, a catalyst may change the rate at which dynamic equilibrium occurs but does not change the value of the equilibrium constant.
3. Write equilibrium constant expressions, Kp, for the following reactions.
(a) CO(g) + H2O(g) → ← CO2(g) + H2(g)
(b) N2(g) + 3 H2(g) → ← 2 NH3(g)
(c) NH4HS(s) → ← NH3(g) + H2S(g)
Use the definition of mass action to write the equilibrium constant expression:
(a)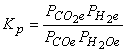
(b)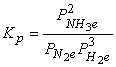
(c)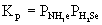
4. Write an equilibrium constant expression, Kp, based on the formation of one mole of each of the following gaseous compounds from its elements at 25 °C.
(a) NO(g) (b) NH3(g) (c) NOCl(g)
In each case, first write the balanced chemical reaction and then use the stoichiometric coefficients in the mass action expression to give the equilibrium constant expression.
(a) ½N2(g) + ½O2(g) → ← NO(g)
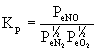
(b) ½N2(g) + 3 / 2H2(g) →← NH3(g)
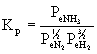
(c) ½N2(g) + ½O2(g) + ½Cl2(g) →← NOCl(g)
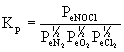
5. Write an equilibrium constant expressions, Kc, for each of the following reversible reactions.
(a) Carbon monoxide gas reduces nitrogen monoxide gas to gaseous nitrogen; carbon dioxide gas is the other product.
(b) Oxygen gas oxidizes gaseous ammonia to gaseous nitrogen monoxide; water vapor is the other product.
(c) Solid sodium hydrogen carbonate decomposes to form solid sodium carbonate, water vapor, and carbon dioxide gas.
Write the balanced chemical reaction and then use the definition of mass action to find the equilibrium constant expression.
(a) 2 CO(g) + 2 NO(g) → ← N2(g) + 2 CO2(g)
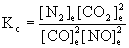
(b) 5 O2(g) + 4 NH3(g) → ← 4 NO(g) + 6 H2O(g)
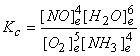
(c) 2 NaHCO3(s) → ← Na2CO3(s) + H2O(g) + CO2(g)
6. In which of the following reactions will the amount of product at equilibrium increase if the total gas pressure is raised from 1 atm to 10 atm? Explain.
(a) SO2(g) + Cl2(g) → ← SO2Cl2(g)
(b) N2(g) + O2(g) → ← 2 NO(g)
(c) SO2(g) + ½ O2(g) → ← SO3(g)
LeChatelier's Principle says that an equilibrium will shift to compensate for any added stress. In this case, the pressure is raised from 1 atm to 10 atm, so each reaction will shift to alleviate that pressure, i.e., towards the side with fewer moles of gases.
(a) Reactants: 2 moles of gases. Products: 1 mole of gases.
An increase in pressure will cause a shift towards products, therefore there will be more product formation at 10 atm.
(b) Reactants: 2 moles of gases. Products: 2 mole of gases.
Both sides have the same number of moles of gases so there will be no shift in the equilibrium.
(c) Reactants: 1 moles of gases. Products: 1.5 mole of gases.
An increase in pressure will cause a shift towards products, therefore there will be more product formation at 10 atm.
7. Determine the values of Kc that correspond to the following values of Kp.
(a) SO2Cl2(g) → ← SO2(g) + Cl2(g)
Kp = 2.9×10–2 at 303 K
(b) 2 NO2(g) → ← 2 NO(g) + O2(g)
Kp = 0.275 at 700 K
(c) CO(g) + Cl2(g) → ← COCl2(g)
Kp = 22.5 at 395 °C
In each case, find the change in the number of moles of gases between product and reactant and then use the relationship Kp = Kc(RT)Δn, where T is in K and R = 0.0821 L·atm/mol·K
(a) Δn = [1 + 1] – [1] = 1
Kp = 2.9×10–2 at 303 K so Kc = Kp/(RT)1 = 2.9×10–2/(0.0821×303)1 = 1.2×10–3
(b) Δn = [2 + 1] – [2] = 1
Kp = 0.275 at 700 K so Kc = Kp/(RT)1 = 0.275/(0.0821×700)1 = 4.8×10–3
(c) Δn = [1] – [1 + 1] = –1
Kp = 22.5 at 395 °C = 668 K so Kc = Kp/(RT)–1 = Kp(RT)1 = 22.5(0.0821×668)1 = 1.23×103
8. Determine the values of Kp that correspond to the following values of Kc.
(a) CO(g) + Cl2(g) → ← COCl2(g)
Kc = 1.2×103 at 668 K
(b) 2 NO(g) + Br2(g) → ← 2 NOBr(g)
Kc = 1.32×10–2 at 1000 K
(c) 2 COF2(g) → ← CO2(g) + CF4(g)
Kc = 2.00 at 1000 °C
Use the relationship Kp = Kc(RT)Δn
(a) Δn = 1 – 2 = –1, so Kp = 1.2×103(0.0821×668)–1 = 22
(b) Δn = 2 – 3 = –1, so Kp = 1.32×10–2(0.0821×1000)–1 = 1.61×10–4
(c) Δn = 2 – 2 = 0, T = 1000 + 273 = 1273 K so Kp = 2.00(0.0821×1273)0 = 2.00
9. For the reaction N2(g) + O2(g) → ← 2 NO(g), Kc = 4.08×10–4 at 2000 K.
What is the value of Kc at 2000 K for the reaction NO(g) → ← ½ N2(g) + ½ O2(g)?
The given reaction is N2(g) + O2(g) → ← 2 NO(g), Kc = 4.08×10–4
Reversing the reaction gives the proper reactants and products for the target reaction, but with the wrong stoichiometry. Reversing the reaction also means that the new equilibrium constant is the inverse of the original equilibrium constant.
2 NO(g) → ← N2(g) + O2(g) Kc = 1/4.08×10–4 = 2.45×103
To obtain the correct stoichiometry for the target reaction, all of the stochiometric coefficients are multiplied by ½. This means that the new equilibrium constant is the square root of the old equilibrium constant.
NO(g) → ← ½ N2(g) + ½ O2(g) Kc = (2.45×103)½ = 49.5
10. Determine Kc at 298 K for the reaction 2 CH4(g) → ← C2H2(g) + 3 H2(g), given the following data at 298 K.
CH4(g) + H2O(g) → ← CO(g) + 3 H2(g) Kp = 1.2×10–25
2 C2H2(g) + 3 O2(g) → ← 4 CO(g) + 2 H2O(g) Kp = 1.1×102
H2(g) + ½ O2(g) → ← H2O(g) Kp = 1.1×1040
(Hint: How are Kc and Kp for the reaction related?)
First, find Kp for the target reaction using the Law of Multiple Equilibria. Then, use the relationship Kp = Kc(RT)Δn to find the value of Kc.
The target reaction has 2 CH4(g) as one of the reactants and 1 C2H2(g) as a product. This tells us that the first reaction must have all of the stoichiometric coefficients doubled and that the Kp for the given reaction must be squared. The stoichiometric coefficients of the second reaction must all be halved and then the reaction reversed so this changes the Kp to the inverse of the square root of the given reaction.
2 CH4(g) + 2 H2O(g) → ←2 CO(g) + 6 H2(g) Kp = (1.2×10–25)2 = 1.4×10–50
2 CO(g) + H2O(g) → ← C2H2(g) + 3 / 2O2(g) Kp = 1/(1.1×102)½ = 9.5×10–2
The sum of these two reactions is given below, which conveniently eliminates CO(g). Kp for the summed reaction is found by multiplying the two equilibrium constants.
2 CH4(g) + 3 H2O(g) → ← C2H2(g) + 6 H2(g) + 3 / 2O2(g) Kp = (1.4×10–50)( 9.5×10–2) = 1.3×10–51
This is not the target reaction. To reach the target, the third reaction given above must be used with all of its stoichiometric coefficents multiplied by three. This means that the equilbrium constant must be cubed.
3 H2(g) + 3 / 2O2(g) → ← 3 H2O(g) Kp = (1.1×1040)3 = 1.3×10120
Now, adding the last two reactions together gives the target reaction. Kp for the target reaction is found by multiplying equilibrium constants.
2 CH4(g) → ← C2H2(g) + 3 H2(g) Kp = (1.3×10–51)( 1.3×10120) = 1.7×1069
Finally, to find Kc for the final reaction, Δn = [1 + 3] – [2] = 2 so Kc = Kp/(RT)Δn = (1.7×1069)/(0.0821×298)2 = 2.8×1066
11. Determine Kc at 298 K for the reaction ½ N2(g) + ½ O2(g) + ½ Cl2(g) → ← NOCl(g), given the following data set at 298 K.
½ N2(g) + O2(g) → ← NO2(g) Kp = 1.0×10–9 (1)
NOCl(g) + ½ O2(g) → ← NO2Cl(g) Kp = 1.1×102 (2)
NO2(g) + ½ Cl2(g) → ← NO2Cl(g) Kp = 0.3 (3)
(Hint: How are Kc and Kp for the reaction related?)
Since the equilibrium constant data is given in terms of Kp for the known reactions, first find Kp for the unknown reaction and then, lastly, convert it to Kc.
The target reaction is:
½ N2(g) + ½ O2(g) + ½ Cl2(g) → ← NOCl(g)
Reaction (1) has nitrogen and oxygen as reactants but a wrong product. Reaction (3) can be used to eliminate the nitrogen dioxide product from (1) and also introduces chlorine:
½ N2(g) + O2(g) → ← NO2(g) Kp = 1.0×10–9 (1)
NO2(g) + ½ Cl2(g) → ← NO2Cl(g) Kp = 0.3 (3)
Adding the two reactions together means that we multiply equilibrium constants:
½N2(g) + O2(g) → ← NO2Cl(g) Kp = (1.0×10–9)(0.3) = 3×10–10 (4)
(The NO2(g) found on both reactants and products sides has been eliminated.)
This has all the correct reactants, but the product is wrong and the stoichiometry is not quite right. If reaction (2) is reversed (meaning Kp is inverted), it can be added to reaction (4) to give the target reaction:
½N2(g) + O2(g) → ← NO2Cl(g) Kp = 3×10–10 (4)
NO2Cl(g) → ← NOCl(g) + ½ O2(g) Kp = (1/1.1×102 = 9.1×10–3 (2rev)
Adding (4) and 2rev gives the desired reaction, both eliminating the NO2Cl and correcting the stoichiometry.
½ N2(g) + ½ O2(g) +
½ Cl2(g)→
← NOCl(g)
With Kp = Kp(4)×Kp(2rev)
= (3×10–10)( 9.1×10–3) = 3×10–12
To find Kc, use the relationship Kp = Kc(RT)Δn
Δn = 1 –(½ + ½ + ½ ) = –½
R = 0.0821 L·atm/mol·K
T = 298 K
so
3×10–12 = Kc[(0.0821)(298)]–½
Kc = 3×10–12[24.5]+½ = 1×10–11
12. If the equilibrium concentrations found in the reaction A(g) + 2 B(g) → ← 2 C(g) are [A] = 0.025 M, [B] = 0.15 M, and [C] = 0.55 M, calculate the value of Kc.
The mass action expression for the reaction is 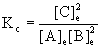 .
.
The equilibrium concentrations of each component is given, so can be directly put into the mass
action expression to give 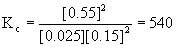 .
.
13. In a sealed 1.75 L vessel at 250 °C, equilibrium is established between PCl5(g) and its dissociation products, PCl3(g) and Cl2(g). The quantities found at equilibrium are 0.562 g PCl5, 1.950 g PCl3, and 1.007 g Cl2. What is the value of Kc for the reaction PCl5(g) → ← PCl3(g) + Cl2(g)? What is the value of Kp?
From the balanced reaction and the definition of Kc we can write:
PCl5(g) → ← PCl3(g) + Cl2(g)
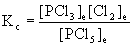
To find Kc, then, we need to find the equilibrium concentration of each component in the reaction from the given masses, the volume of the container, and the molar mass of each species.
PCl5: [30.97 + 5(35.45)] = 208.22 g/mol. 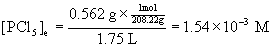
PCl3: [30.97 + 3(35.45)] = 137.32 g/mol. 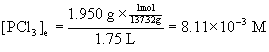
Cl2: [2(35.45)] = 70.90 g/mol. 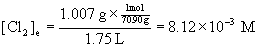
Plugging each of these concentrations into the expression for Kc gives:
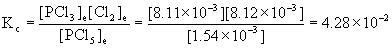
Kp = Kc(RT)Δn, Δn = (1+1) – 1 = 1; R = 0.0821 L·atm/mol·K; T = 250 + 273 = 523 K
Kp = 4.28×10–2[(0.0821)(523)]1 = 1.84
14. Ammonium hydrogen sulfide dissociates into ammonia gas and hydrogen sulfide gas. If we start with a sample of pure NH4HS(s) at 25 °C, the total pressure of the gases is 0.658 atm when equilibrium is established. Write an equation for the dissociation reaction and determine the value of Kp
Set up an ICE table:
NH4HS(s) → ← NH3(g) + H2S(g)
Kp = PNH3(e)PH2S(e)
Initial? 0 0
Change–x +x +x
Equilibrium–x x x
In this case x represents the partial pressure of the gases in the container and the total pressure in the container is equal to the sum of the partial pressures (Dalton's Law of Partial Pressures). Thus, 0.658 atm = x + x = 2x, or x = 0.329 atm.
Now, Kp = (x)(x) = x2 = (0.329)2 = 0.108.
15. The equilibrium constant for the isomerization of butane at 25 °C is Kc = 7.94.
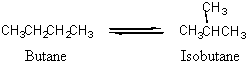
If 5.00 g butane is introduced into a 12.5 L flask at 25 °C, what mass of isobutane will be present when equilibrium is reached?
Convert the mass of butane (abbreviated B) to molarity, then set up a table of concentrations to solve for the equilibrium concentration of isobutane (abbreviated i-B). Then convert this molarity back to a mass of isobutane.
B: C4H10 molar mass = 4(12.01) + 10(1.01) = 58.14 g/mol
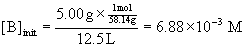
B(g) → ← i-B(g)
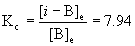
Initial6.88×10–3 0
Change–x +x
Equilibrium6.88×10–3 – x x
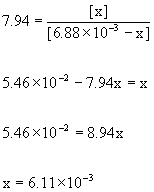
[i-B]e = 6.11×10–3 M
i-B: C4H10 so the molar mass is the same as butane (which must be true if they are isomers) = 58.14 g/mol.
So the mass of isobutane at equilibrium is:
mass = Molarity×Volume×molar mass = 6.11×10–3 mol/L × 12.5 L × 58.14 g/mol = 4.44 g
16. For the water-gas reaction {C(s + H2O(g → ← CO(g) + H2(g)], Kc = 0.111 at about 1100 K.
If 0.100 mol H2O(g) and 0.100 mol H2(g) are mixed with excess C(s) at this temperature and equilibrium is established, how many moles of CO(g) will be present? No CO(g) is present initially.
A typical equilibrium problem: write the reaction, write the mass action expression, set up a table of concentrations, then plug into the mass action expression and solve. Assume a 1.00 L reaction vessel.
C(s) + H2O(g) → ← CO(g) + H2(g)

Initialxs 0.100 0 0.100
Change – x +x +x
Equilibrium 0.100 – x x 0.100 + x
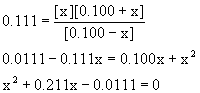
This is solved using the quadratic equation to give
x = 0.0436 or x = –0.255
Since x must be a positive number x = [CO] = 0.0436 M = 0.0436 mol in a 1.00 L vessel.
Note: because Δn = +1 for this reaction, the number of moles of CO present at equilibrium depends upon the size of the reaction vessel. For example, if you do the problem assuming a 2.00 L vessel, the amount of CO present at equilibrium is 0.0584 mol.
17. For the reaction CO(g) + H2O(g) → ← CO2(g) + H2(g), Kc = 23.3 at 600 K.
If 0.250 mol each of CO and H2O are introduced into a reaction vessel and equilibrium is established, how many moles each of CO2 and H2 will be present at equilibrium? (Hint: Does the volume of the reaction mixture matter?)
Set up a table of concentrations and solve from there.
CO(g) + H2O(g) → ← CO2(g) + H2(g)
Initial0.250 0.250 0 0
Change– x – x +x +x
Equilibrium0.250 – x 0.250 – x x x
Note that the volume of the container does not matter in this problem (this is not always true):
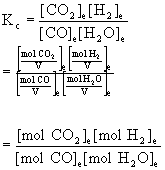
Because the stoichiometry of the reactants and products is the same, the volume terms cancel out.
To solve the problem:
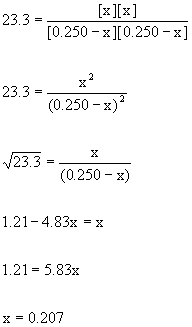
So the number moles of CO2 = the number of moles of H2 = 0.207 mol
18. To establish equilibrium in the following reaction at 250 °C,
PCl3(g) + Cl2(g) → ← PCl5(g) Kc = 26 at 250 °C
0.100 mol each of PCl3 and Cl2 and 0.0100 mol PCl5 are introduced into a 6.40 L reaction flask. How many moles of each of the gases will be present when equilibrium is established?
First, convert the initial amounts of species from moles to molarity. Then do the usual equilibrium problem. Finally, convert the calculated molarities back to moles to obtain the answers.
PCl3(g) + Cl2(g) → ← PCl5(g)
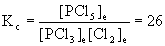
Initial0.100/6.40 = 0.0156 0.100/6.40 = 0.0156 0.0100/6.40 = 0.00156
Change– x – x +x
Equilibrium0.0156 – x 0.0156 – x 0.00156 + x
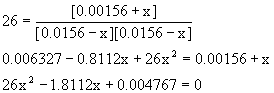
Solving with the quadratic equation gives
x = 0.067 or x = 0.0027 (2 significant figures because of Kc)
Since x must be less than 0.0156 (so that the PCl3 and Cl2 concentrations are positive at equilibrium), the correct value for x is 0.0027.
Thus, [PCl3]e = 0.0156 – 0.0027 = 0.0129 M, [Cl2]e = 0.0156 – 0.0027 = 0.0129 M, and [PCl5]e = 0.00156 + 0.0027 = 0.0043 M.
The number of moles of each component is found by multiplying each molarity by the volume of the reaction flask:
PCl3: 0.0129×6.40 = 0.0826 mol
Cl2: 0.0129×6.40 = 0.0826 mol
PCl5: 0.0043×6.40 = 0.028 mol
19. An analysis of the gaseous phase [S2(g) and CS2(g)] present at equilibrium at 1009 °C in the reaction
C(s) + S2(g) → ← CS2(g)
shows it to be 13.71% C and 86.29% S, by mass. What is Kc for this reaction?
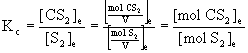 , i.e., the volumes cancel for this reaction.
, i.e., the volumes cancel for this reaction.
Since volume is unimportant for finding the equilibrium constant, suppose the volume of gas chosen
for analysis contained 100.0 g of sample. This means that of the 100.0 g, 13.71 g of the sample was C and 86.29 g
of the sample was S, based on the analysis. The number of moles of C in the gas phase sample = 13.71 g / 12.011 g/mol = 1.141 mol.
All of the carbon in the gas phase is in the carbon disulfide, so the 100.0 g sample must contain 1.141 mol CS2.
The total number of moles of sulfur in the 100.0 g sample = 86.29 g / 32.066 g/mol = 2.691 mol. Of this total, 2(1.141) = 2.282 mol
of sulfur is used in CS2, leaving 2.691 – 2.282 = 0.409 mol sulfur in S2, or 0.409/2 = 0.205 mol S2.
Thus, at equilibrium, in the 100.0 g sample there is 1.141 mol CS2 and 0.205 mol S2. Now Kc can be found:
Kc = (1.141)/(0.205) = 5.57.