 Practice Problems
Chemical Kinetics: Rates and Mechanisms of Chemical Reactions
Practice Problems
Chemical Kinetics: Rates and Mechanisms of Chemical Reactions Practice Problems
Chemical Kinetics: Rates and Mechanisms of Chemical Reactions
Practice Problems
Chemical Kinetics: Rates and Mechanisms of Chemical Reactions1. State two quantities that must be measured to establish the rate of a chemical reaction and cite several factors that affect the rate of a chemical reaction.
2. Explain why the rate of disappearance of NO and the rate of formation of N2 are not the same in the reaction, 2CO(g) + 2NO(g) → 2CO2(g) + N2(g).
3. What plot of experimental data can be used to evaluate the activation energy, Ea, of a reaction? How is Ea related to this plot?
4. What are the chief requirements that must be met by a plausible reaction mechanism? Why do we say "plausible" mechanism rather than "correct" mechanism?
5. In a reaction mechanism, (a) what is the difference between an activated complex and an intermediate? (b) What is meant by the rate-determining step? Which elementary reaction in a reaction mechanism is often the rate-determining step?
6. In the reaction H2O2(aq) → H2O(l) + ½ O2(g), the initial concentration of H2O2 is 0.2546 M, and the initial rate of reaction is 9.32×10–4 M s–1. What will be [H2O2] at t = 35 s?
7. For the reaction, A → products, a graph of [A] versus time is a curve. What can be concluded about the order of this reaction?
8. Following are two statements pertaining to the reaction 2A + B → 2C, for which the rate law is rate = k[A][B]. Identify which statement is true and which is false, and explain your reasoning.
(a) The value of k is independent of the initial concentrations [A]0 and [B]0.
(b) The unit of the rate constant for this reaction can be expressed either as s–1 or min–1.
9. The rate of the following reaction in aqueous solution is monitored by measuring the number of moles of Hg2Cl2 that precipitate per liter per minute. The data obtained are listed in the table.
2 HgCl2(aq) + C2O42–(aq) → 2 Cl–(aq) + 2 CO2(g) + Hg2Cl2(s)
Experiment [HgCl2] (M) [C2O42–] (M) Initial rate (mol L–1 min–1)
1 0.105 0.15 1.8×10–5
2 0.105 0.15 1.8×10–5
3 0.052 0.30 7.1×10–5
4 0.052 0.15 8.9×10–6
(a) Determine the order of reaction with respect to HgCl2, with respect to C2O42– and overall.
(b) What is the value of the rate constant k?
(c) What would be the initial rate of reaction if [HgCl2] = 0.094 M and [C2O42–] = 0.19 M?
(d) Are all four experiments necessary to answer parts (a) - (c)? Explain.
10. In the reaction A → products, we find that when [A] has fallen to half of its initial value, the reaction proceeds at the same rate as its initial rate. Is the reaction zero order, first order, or second order? Explain.
11. In the reaction, A → products, with the initial concentration [A]0 = 1.512 M, [A] is found to be 1.496 M at t = 30 s. With the initial concentration [A]0 = 2.584 M, [A] is found to be 2.552 M at t = 1 min. What is the order of this reaction?
12. A first order reaction, A → products, has a rate of reaction of 0.00250 M s–1 when [A] = 0.484 M. (a) What is the rate constant, k, for this reaction? (b) Does t3/4 depend on the initial concentration? Does t4/5? Explain.
13. In the first-order decomposition of dinitrogen pentoxide at 335 K,
N2O5(g) → 2 NO2(g) + ½ O2(g)
if we start with a 2.50-g sample of N2O5 at 335 K and have 1.50 g remaining after 109 s, (a) What is the value of the rate constant k? (b) What is the half-life of the reaction? (c) What mass of N2O5 will remain after 5.0 min?
14. The smog constituent peroxyacetyl nitrate (PAN) dissociates into peroxyacetyl radicals and NO2(g) in a first order reaction with a half-life of 32 min.
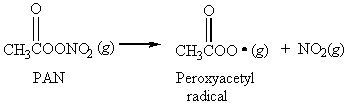
If the initial concentration of PAN in an air sample is 2.7×1015 molecules/L, what will be the concentration 2.24 h later?
15. The following data were obtained in two separate experiments in the reaction: A → products. Determine the rate law for this reaction, including the value of k.
Experiment 1 Experiment 2
[A] (M) time (s) [A] (M) time (s)
0.800 0 0.400 0
0.775 40 0.390 64
0.750 83 0.380 132
0.725 129 0.370 203
0.700 179 0.360 278
16. Listed below are initial rates, expressed in terms of the rate of decrease of partial pressure of a reactant for the following reaction at 826 °C. Determine the rate law for this reaction, including the value for k.
NO(g) + H2(g) → ½N2(g) + H2O(g)
With initial PH2 = 400 mmHg With initial PNO = 400 mmHg
Initial PNO (mmHg) Rate (mmHg/s) Initial PH2 (mmHg) Rate (mmHg/s)
359 0.750 289 0.800
300 0.515 205 0.550
152 0.125 147 0.395
17. Rate constants for the first-order decomposition of acetonedicarboxylic acid
CO(CH2COOH)2(aq) → CO(CH3)2(aq) + 2 CO2(g)
acetonedicarboxylic acidacetone
are k = 4.75 ×10–4 s–1 at 293 K and k = 1.63 ×10–3 at 303 K. What is the activation energy, Ea, for this reaction?
18. The following is proposed as a plausible reaction mechanism:
A + B → I(slow)
I + B → C + D(fast)
What is (a) the net reaction described by this mechanism and (b) a plausible rate law for the reaction?
19. The following reaction exhibits the rate law: Rate = k[NO]2[Cl2].
2 NO(g) + Cl2(g) → 2 NOCl(g)
Explain why the following mechanism is not plausible for this reaction.
NO(g) + Cl2 → ← NOCl(g) + Cl(g)fast
NO(g) + Cl(g) → NOCl(g)slow
20. Show that the proposed mechanism is consistent with the rate law for the following reaction in aqueous solution,
Hg22+(aq) + Tl3+(aq) → 2 Hg2+(aq) + Tl+(aq)
for which the observed rate law is
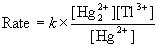
Proposed Mechanism:
Hg22+(aq) → ← Hg2+(aq) + Hg(s)fast
Hg(s) + Tl3+(aq) → Hg2+(aq) + Tl+(aq) slow
21. Benzenediazonium chloride decomposes in water yielding N2(g).
C6H5N2Cl(aq) → C6H5Cl(aq) + N2(g)
The data tabulated below were obtained for the decomposition of a 0.071 M solution at 50 °C (t = ∞ corresponds to the completed reaction). To obtain [C6H5N2Cl] as a function of time, note that during the first 3 min, the volume of N2(g) produced was 10.8 mL of a total of 58.3 mL, corresponding to this fraction of the total reaction: 10.8 mL/58.3 mL = 0.185. An equal fraction of the available C6H5N2Cl was consumed during the same time.
time (min)N2(g) (mL)
00
310.8
619.3
926.3
1232.4
1537.3
1841.3
2144.3
2446.5
2748.4
3050.4
∞58.3
(a) Plot graphs showing the disappearance of C6H5N2Cl and the formation of N2(g) as a function of time.
(b) What is the initial rate of formation of N2(g)?
(c) What is the rate of disappearance of C6H5N2Cl at t = 20 min?
(d) What is the half-life, t½, of the reaction?
(e) Write the rate law for this reaction, including a value for k.
22. Hydroxide ion is involved in the mechanism but not consumed in this reaction in aqueous solution.
OCl–(aq) + I–(aq) → OH– OI–(aq) + Cl–(aq)
(a) From the data in the table, determine the order of reaction with respect to OCl–, I–, and OH–, and the overall order.
[OCl–] (M)[I–] (M) [OH–] (M) Rate of formation of OI– (mol L–1 s–1)
0.00400.0020 1.00 4.8×10–4
0.00200.0040 1.00 5.0×10–4
0.00200.0020 1.00 2.4×10–4
0.00200.0020 0.50 4.6×10–4
0.00200.0020 0.25 9.4×10–4
(b) Write the rate law, and determine the value of the rate constant, k.
(c) Show that the following mechanism is consistent with the net equation and with the rate law. Which is the rate–determining step?
OCl –(aq) + H2O(l) → ← HOCl(aq) + OH –(aq)
I –(aq) + HOCl(aq) → HOI(aq) + Cl –(aq)
HOI(aq) + OH –(aq) → H2O(l) + OI –(aq)
(d) Is it appropriate to refer to OH–as a catalyst in this reaction? Explain.