
From thermodynamics, recall
ΔG = ΔG° + RTln Q
If we divide everything by –nF
ΔG –nF = ΔG° –nF + RT –nF ln Q
since ΔG = –nFE, or E = ΔG/(–nF)
E = E° – RT nF ln Q
R = 8.314 J/mol·K (the gas constant)
F = 96485 coul/mol (Faraday's constant)
T = absolute temperature
n = number of moles of electrons transferred in the balanced equation
Q = reaction quotient
This equation is known as the Nernst Equation and can be used to find a cell potential at any set of conditions.
Consider the Daniell Cell at 25 °C
Zn(s) + Cu2+(aq) → Cu(s) + Zn2+(aq)
Find the cell potential at the following conditions when [Cu2+] = 1.00 M, [Zn2+] = 1.0×10–9 M and when [Cu2+] = 0.10 M, [Zn2+] = 0.90 M.
Recall that the standard potential for the Daniell cell is E° = +1.10 V
Then, the Nernst equation can be used to find the potentials at the nonstandard conditions:
E = E° – RT nF ln Q
Q = [Zn2+]/[Cu2+]
E = E° – RT nF ln ([Zn2+]/[Cu2+])
E = +1.10 – (8.314)(298) (2)(96485) ln ([Zn2+]/[Cu2+])
E = 1.10 – 0.0128 ln ([Zn2+]/[Cu2+])
This is the working equation specific to this problem.
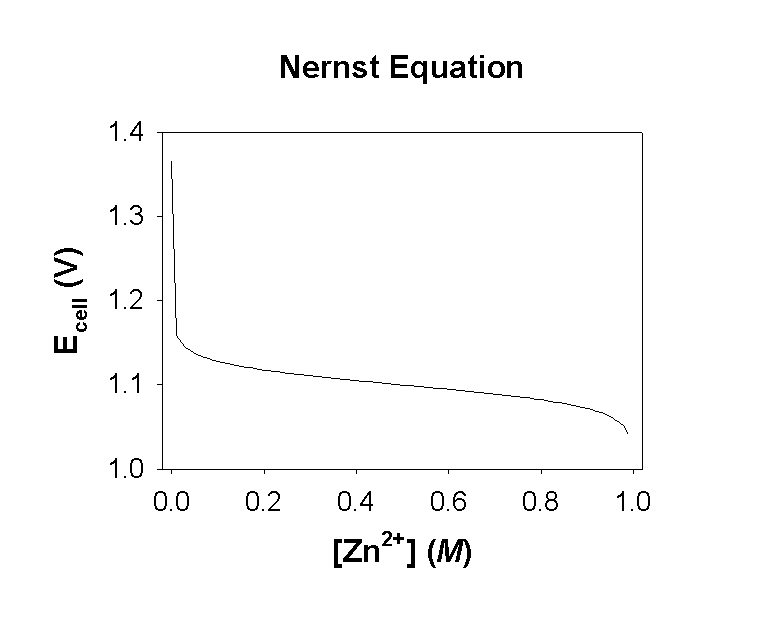
When [Cu2+] = 1.00 M, [Zn2+] = 1.0×10–9 M so E = +1.10 – 0.0128 ln(1.0×10–9/1.00) = 1.37 V
When [Cu2+] = 0.10 M, [Zn2+] = 0.90 M so E = +1.10 – 0.0128 ln(0.90/0.10) = 1.07 V
A plot of the cell potential versus the reaction progress is shown below:
Consider the cell:
Pt(s)|OH–(aq)(pH = 13.0)|O2(g)(0.2 atm)||O2(g)(0.2 atm)|H+(aq)(pH = 1.0)|Pt(s)
What are the cell potentials at 25 , 37, and 50 °C?
Anode (oxidation) reaction:
4 OH–(aq) → O2(g) + 2 H2O(l) + 4 e–
E°ox = –0.401 V
Cathode (reduction) reaction:
O2(g) + 4 H+(aq) + 4 e– → 2 H2O(l)
E°red = +1.229 V
Net reaction:
4 H+(aq) + 4 OH–(aq) → 4 H2O(l)
OR:
H+(aq) + OH–(aq) → H2O(l)
E° = –0.401 + 1.229 = +0.828 V
E = E° – RT nF ln Q
Q = Qanode×Qcathode
Q = PO2anode [OH–]4anode × 1 PO2cathode [H+]4cathode
PO2anode = PO2cathode = 0.2 atm
[H+]cathode = [OH–]cathode = 0.1 M
Q = (0.2)/(0.10)4(0.2)(0.1)4 = 1×108
E = +0.828 – (8.314)T (4)(96485) ln (1×108)
E = 0.828 – 0.000397T
T (°C)T (K) E (V)
25298 +0.710
37310 +0.705
50323 +0.700
The temperature dependence of cell voltages is typically quite small.