
Electrochemistry is the study of chemical reactions by use of electrical circuits. This is a natural way to investigate oxidation/reduction reactions since there is an electron transfer occurring. By forcing the transferred electrons to move through a wire, we can measure reaction progress (kinetics), composition (equilibrium constants), energy changes (thermodynamics), and add or subtract energy from the system.
A typical electrochemical cell:
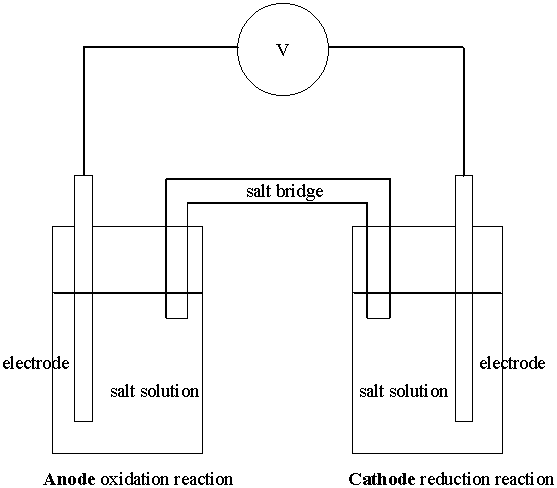
The oxidation and reduction halfreactions are physically separated and connected by an electrical circuit.
The electrical circuit is completed by using two different types of connections.
The wire: this is the path that electrons flow from the oxidation to the reduction.
The salt bridge: this completes the electrical circuit but does not allow electron flow. Rather, the charge is carried by ions (cations or anions). The salt bridge also prevents the two reacting solutions from mixing.
The electrode where oxidation occurs is called the anode.
The electrode where reduction occurs is called the cathode.
If the net oxidation/reduction reaction is spontaneous, the cell is called a voltaic cell or a galvanic cell.
Useful work can be done by voltaic cells (these are batteries).
If the net oxidation/reduction reaction is nonspontaneous, the cell is called an electrolytic cell. Energy must be supplied to an electrolytic cell in order to drive the oxidation/reduction reaction.
anode reaction||cathode reaction
The double bars (||) represent the salt bridge
The essential components of each half reaction are described and each piece of information is separated by a single bar (|), which also represents a phase boundary. This may include the nature of the electrode, phase information, concentrations or partial pressures, or temperature if the cell is not at standard conditions.
Write the half-reactions and net reaction for the cell Al(s)|Al3+(aq)||Hg2+(aq)|Hg(l)|Pt(s)
Anode (oxidation) reaction:
Al(s) → Al3+(aq) + 3 e–
The solid aluminum serves as the physical electrode.
Cathode (reduction) reaction:
Hg2+(aq) + 2 e– → Hg(l)
The solid platinum serves as the physical electrode.
Net reaction:
2 Al(s) + 3 Hg2+(aq) → 2 Al3+(aq) + 3 Hg(l)
Voltaic cells are used to convert chemical energy to electrical energy that can be used to produce useful work.
Physicists teach us that electrical work is found by multiplying the charge by the potential difference:
w = –charge×potential difference
charge is measured in coulombs, so the total amount of charge that can be produced from an electrochemical cell is
charge = coulombs moles electrons × moles electrons
Each mole of electrons produces 96485 coulombs; this is known as Faraday's constant:
F = 96485 coul/mol
The difference in potential between is electrodes is the cell voltage = Ecell
If the number of moles of electrons is represented by n, then
w = –nFEcell
The potential of a cell depends upon the amount of current flowing, but under open circuit conditions the potential is maximum, so
wmax = –nFEmax
Emax = Eopen circuit is normally just labeled Ecell
Recall that the maximum work is found from the Gibb's Energy:
ΔG = wmax = –nFEcell
Standard-state conditions for electrochemical cells are defined as: all solutes having a concentration of 1 M and all gas having a partial pressure of 1 bar (~1 atm).
At standard-state conditions:
ΔG° = –nFE°cell
Since voltaic cells are spontaneous, the potential must be positive.
We would like to be able to predict cell potentials for any reaction using data from tables. We do this by assigning a potential to half-reactions. Then, by combining the half-reaction potentials in the combination found in an experimental cell, we can determine the cell potential:
E°cell = E°reduction + E°oxidation
There is one problem with this approach – setting the scale. Each experimental cell provides a single potential measurement but we want to partition it into two parts. How do we do this consistently?
We choose a reference reaction:
2 H+(aq) + 2 e– → H2(g)
at standard conditions ([H+] = 1.00 M, P(H2(g)) = 1.00 atm, T = 298 K), the potential for this half reaction is defined to be
E°reduction = 0.000 V
Now a series of cells can be measured; for example:
Ni(s)|Ni2+(aq)||H+(aq)|H2(g)|Pt
Cu(s)|Cu2+(aq)||H+(aq)|H2(g)|Pt
Zn(s)|Zn2+(aq)||H+(aq)|H2(g)|Pt
Na(s)|Na+(aq)||H+(aq)|H2(g)|Pt
etc.
In each case, the measured cell potential gives the oxidation potential for the anode reaction.
Further, cell potentials are thermodynamic quantities. This means that if we reverse the direction of a reaction, the sign of the potential changes but not the magnitude.
For example:
Cu(s) → Cu2+(aq) + 2 e– E°oxidation = –0.34 V
Cu2+(aq) + 2 e– → Cu(s) E°reduction = +0.34 V
Thus, all half-cell potentials are reported as reduction potentials and are listed in a Table of Standard Reduction Potentials.
The Table of Standard Reduction Potentials gives us two pieces of information:
The balanced half-reaction.
The standard reduction potential.
Find the cell potential for the Daniell cell:
Cu2+(aq) + Zn(s) → Cu(s) + Zn2+(aq)
The reduction half-reaction is:
Cu2+(aq) + 2 e– → Cu(s)
E°reduction = +0.34 V
The oxidation half-reaction is:
Zn(s) → Zn2+(aq) + 2 e–
E°reduction(Zn2+) = –0.76 V, so E°oxidation = +0.76 V
Then, the cell potential is
E° = (+0.34) + (+0.76) = +1.10 V
Another way to think about this is to write:
E° = E°cathode – E°anode
where E°cathode and E°anode are standard reduction potentials taken directly from the Table of Standard Reduction Potentials, i.e. E°oxidation = –E°anode