
Consider the following reaction at 500 K:
CH3NO2(g) → products
Suppose the following data were measured:
time (s)[CH3NO2] (mol/L)
00.200
3000.145
6000.105
9000.076
12000.055
How fast is the reaction proceeding?
Or, more precisely asked, what is the rate of the reaction?
According to the definition
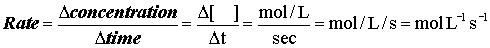
Rates at different times:
Between 0 and 300 s
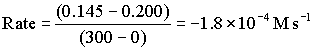
Between 300 and 600 s
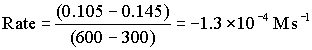
Between 600 and 900 s

Between 900 and 1200 s
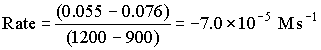
The rate continually decreases as the reaction proceeds.
Note on the sign of the rate: mathematically, the sign of the rate is negative when based on reactants (the change in reactants, Δ[ ] will always be negative since the amount of reactants is decreasing). When the rate is determined based on products, the rate is positive in sign (the amount of products is increasing). Frequently, the sign of the rate is ignored and inferred by context.
Rates also can be different when found from different chemical species because of stoichiometry. The general rate is found by dividing the specific rate by the stoichiometric coefficient.
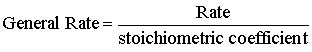
Note that the rates are different. Is this because of experimental problems?
Plot the data: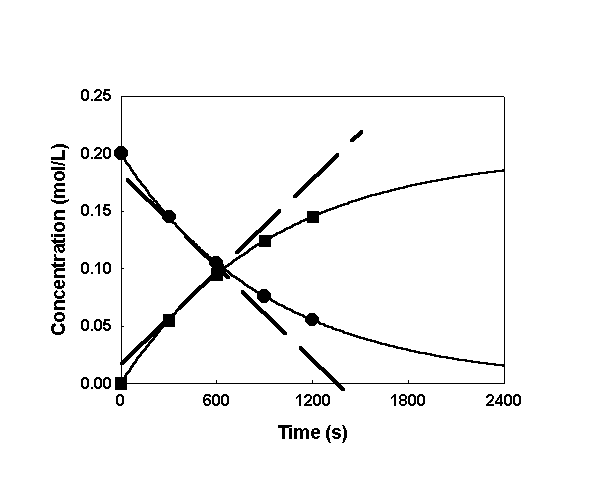
The definition of rate is a slope (indicated as dashed lines). The rate can be found using either the decrease in concentration of reactants (circles) or the increase in concentration of products (squares).
Clearly, the slope changes with time - consistent with what was found earlier.
How do we deal with this?
Consider the general reaction: A + B + C + ... → products
For this reaction: Rate = k[A]m[B]n[C]p...
This is called the Rate Law for the reaction.
k = rate constant
m = order of reaction in reactant A
n = order of reaction in reactant B
p = order of reaction in reactant C
The total order of reaction is the sum of the orders of reaction for each reactant total order = m + n + p + ...
Properties of Rate Laws:
Orders of reaction need not be integers
Orders of reaction need not be positive
Catalyst concentrations may be part of a rate law
Rate Laws are constant at any given temperature
Rate laws must be found experimentally; there is no information in a balanced reaction about the correct form of the rate law.
2 H2(g) + 2 NO(g) → N2(g) + 2 H2O(g)
The experimental rate law is found to be
Rate = k[H2][NO]2
What are the orders of reaction in each reactant? What is the total order of reaction?
The rate of a reaction is found just at the beginning of the reaction. This is done for several, carefully chosen, concentrations of reactants. Then, by application of the rate law at each concentration, the orders of reaction and the rate constant can be found.
Consider the reaction:
A + B → products
Conduct three experiments
[A]1, [B]1, measure Rate1
[A]1, [B]2, measure Rate2
[A]2, [B]1, measure Rate3
From the definition of a rate law:
Rate1 = k[A]1m[B] 1n
Rate2 = k[A]1m[B]2n
Divide the two rates:

Rate1, Rate2, [B]1, and [B]2 are known so simple algebra gives the order of reaction in reactant B
Similarly: 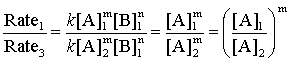
so the order in reactant A is found.
Finally, the rate constant is found by 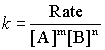
using any (or, better, all) of the experiments and the values of m and n just determined.