
2 NO(g) → ← N2(g) + O2(g)
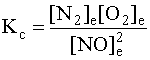
Initial0.100/2.00 = 0.0500 M 0 0
Change–2x +x +x
Equilibrium0.0500 – 2x x x
The final conditions for the experiment give us [NO]e = 0.00044 mol/2.00 L = 0.00022 M.
This allows us to find x:
[NO]e = 0.0500 – 2x = 0.00022
or x = 0.0249 M = [N2]e = [O2]e
Now we have all the values we need to calculate Kc:
Recall that the relationship between Kp and Kc is
Kp = Kc(RT)Δn
Δn = 1 + 1 - 2 = 0
so
Kp = Kc(RT)0 = Kc = 1.3×104