
To determine an equilibrium constant, the concentrations of all the reactants and products at equilibrium must be established. When these are known, then they can be used in the mass action expression to calculate the equilibrium constant at that temperature.
At 1500 °C the following reaction was run:
2 NO(g) → ← N2(g) + O2(g)
0.100 moles of NO were added to a 2.00 L evacuated vessel. At equilibrium, 0.00044 moles of NO were remaining. What are the values of Kc and Kp?
Strategy: the natural units of the problem are M (moles and liters are given) so find Kc first, then use the relationship between Kc and Kp to find Kp. To find Kc, we need to establish the concentrations of each species at the equilibrium condition.
We can do this by setting up a table of concentrations. We know the initial condition and the reaction stoichiometry. This allows us to establish the equilibrium composition in relation to each component.
Sometimes a series of chemical equilibria are related, i.e., two or more reactions add up to a final reaction.
In this situation, the equilibrium constants are related:
If equilibrium reactions can be added to give a total reaction, then the equilibrium constant for the total reaction is equal to the product of the equilibrium constants for the contributing reactions.
Mathematically, this can be written as:
A1(g) + B1(g) + ... → ← C1(g) + D1(g) + ... Kc1
A2(g) + B2(g) + ... → ← C2(g) + D2(g) + ... Kc2
A3(g) + B3(g) + ... → ← C3(g) + D3(g) + ... Kc3
etc.
A1(g) + A2(g) + A3(g) + B1(g) + B2(g) + B3(g) + ... → ← C1(g) + C2(g) + C3(g) + D1(g) + D2(g) + D3(g) + ... Kctotal = Kc1×Kc2×Kc3×...
Find the equilibrium constant for the reaction:
2 N2O(g) + 3 O2(g) → ← 4 NO2(g)
given the following information:
N2O(g) + ½ O2(g) → ← 2 NO(g) Kc = 1.7×10–13 (1)
NO(g) + ½ O2(g) → ← NO2(g) Kc = 6.83×106 (2)
This is a multiple equilibria problem. The goal is to use the reactions with the given equilibrium constants to generate the reaction whose equilibrium constant is unknown.
The target reaction is
2 N2O(g) + 3 O2(g) → ← 4 NO2(g)
Reaction (1) has the correct reactants but with the wrong stoichiometry (for the target) and the wrong products:
N2O(g) + ½ O2(g) → ← 2 NO(g)
In order to get the N2O stoichiometry correct, we need to use (1) again:
N2O(g) + ½ O2(g) → ← 2 NO(g)
Summing these two reactions gives:
2 N2O(g) + 1 O2(g) → ← 4 NO(g)
Reaction (2) can be used to eliminate the NO product that does not show up in the final, target reaction. However, we need to use it four times in order to completely eliminate the NO:
NO(g) + ½ O2(g) → ← NO2(g)
NO(g) + ½ O2(g) → ← NO2(g)
NO(g) + ½ O2(g) → ← NO2(g)
NO(g) + ½ O2(g) → ← NO2(g)
Now the sum of all of these reactions is:
2 N2O(g) + 3 O2(g) + 4 NO(g) → ← 4 NO2(g) + 4 NO(g)
The 4 NO(g) can be eliminated from both sides of the reaction to give the desired reaction.
Then, the equilibrium constant is found by multiplying the equilibrium constants from each contributing reaction:
Kc = Kc1 × Kc1 × Kc2 × Kc2 × Kc2 × Kc2
= (1.7×10–13)(1.7×10–13)(6.83×106)(6.83×106) (6.83×106)(6.83×106) = 63
Consider the following reactions:
SO3(g) + H2O(g) → ← H2SO4(g) (3)
SO3(g) + H2O(l) → ← H2SO4(aq) (4)
Are the equilibrium constants for these two reactions the same?
Kc(3) will equal Kc(4) as long as the following conditions are met:
[H2SO4(g)]e = [H2SO4(aq)]e
and
[H2O(g)]e = [H2O(l)]e
The first condition is possible: the gas phase concentration of sulfuric acid can be a wide range of values, depending upon the partial pressure of the gas; likewise, the number of moles of sulfuric acid dissolved in water can easily be changed so the concentration can attain many values.
The second condition is more demanding. As with the sulfuric acid, the gas phase concentration of water can have many values, depending upon the partial pressure.
However, the liquid phase only has one concentration:
The density of water is 1 g/mL = 1000 g/L
1000 g/18 g/mole = 55.6 moles
so the concentration of liquid water is 55.6 M.
Since the density of water is unchanged by how much is present (1 L of water has the same density as 1 mL of water!), the concentration never changes.
This is always true for pure liquids and pure solids: the concentration (in M) is independent of the amount of material present.
Therefore, the two equilibrium constants are not the same in the above example.
Because pure liquids and pure solids have invariant concentrations, they are not used in mass action expressions. Thus, the proper mass action expression for reaction 4 is
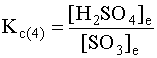
All reactions must show the phase of matter for each reactant and product. This is the only way we can know the correct mass action expression and the correct equilibrium constant.