Speciation of the Products of and Establishing the Role of Water in the Reaction of TNT with Hydroxide and Amines: Structure, Kinetics, and Computational Results
Christopher A. Latendresse, Syrena C. Fernandes, Sangmin You, William B. Euler, J. Phys. Chem. A, 2013, 117, 11167 – 11182
Abstract
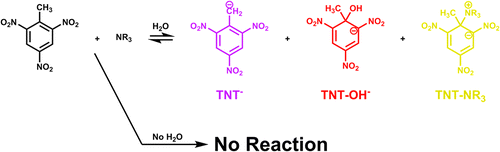
The reaction of trinitrotoluene (TNT) with bases is investigated by NMR and visible spectroscopy methods. Hydroxide ion is found to react in one of two ways, either by deprotonation of the methyl group or by nucleophilic attack on the aromatic ring to form a σ-adduct. The rate of each mode of reaction depends upon the polarity of the solvent. In tetrahydrofuran (THF), σ-adduct formation is rapid and the long term equilibrium product is deprotonation of the methyl group. When the solvent is methanol (MeOH) both reactions have similar rates and the σ-adduct becomes the majority product. Amines are found to be ineffective in directly deprotonating TNT or in forming σ-adducts. Rather, the amines react with ambient water to generate hydroxide ion, which then reacts with TNT. Solvent choice and water content are crucial to understanding the reactivity of bases with TNT. To assist in the interpretation of the experimental results, computational analysis was performed at the B3LYP/6-311+G**//HF/6-311+G** level to determine the thermodynamics of the reactions of TNT. The SM8 implicit solvation model was applied to converged geometries, which suggests a strong solvation effect upon product formation. Thermodynamic analysis suggests a significant preference of alkoxide or hydroxide attack versus amine attack in any modeled dielectric, consistent with the experimental observations.