Mesophase Formation in Regioregular Poly(3-alkylthiophene)s Containing Long Chain Alkyl Groups
Yu Wang, Nadia Archambault, Allison M. Belcher, Devin Busse, David B. Damon, Ashley Mills, Amanda E. Riddle, Ivan J. Samardjiev, Brett L. Lucht, William B. Euler, Macromolecules, 2008, 41, 7115 – 7121
Abstract
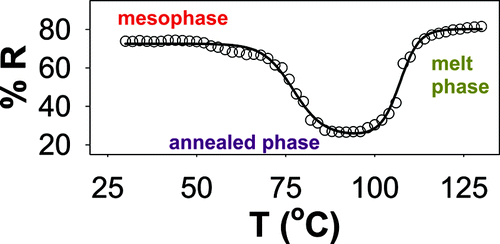
A series of regioregular poly(3-alkylthiophene)s with substituent groups R = C22H45, C24H49, C26H53, and C28H57 were synthesized. All of the materials can form a mesophase by rapid quenching from the isotropic melt. The ease of mesophase formation depends upon the length of the alkyl group, with the longer side chains leading to mesophase formation at lower quenching rates. Variable temperature reflection and fluorescence spectroscopy of thin films and differential scanning calorimetry and variable temperature X-ray diffraction on powders were used to study the thermal behavior of the new polymers. All of the materials studied showed a two-step thermochromic transition from the mesophase, and endotherms in the thermograms could be assigned to melting of each phase. The data indicate that π-π stacking is an important contributor to the thermochromism observed in these compounds while the interaction between the alkyl side chains controls mesophase formation.