Configurational and Conformational Mobility in a Cross Conjugated Derivative of 1,3-Diketo-2-iminopropane
Yueyang Song, Lynne Spencer, William B. Euler, William Rosen, Organic Letters, 1999, 1, 561 – 564
Abstract
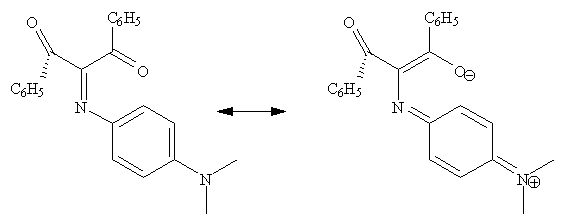
Mobility of configuration and conformation in the "push-pull" cross-conjugated derivative 1,3-diphenyl-2(p-dimethylaminophenyl)-iminopropane-1,3-dione has been investigated by NMR. The energetics of the thermal process that interconnects the syn- and anti-configurations of the imino-group with the atropisomeric conformations of the skewed benzoyl-groups are defined. It is proposed that a symmetric Y-delocalized transition state thermally interconnects the inversion of the imino-configurations with the mobile interchange of the skewed benzoyl-conformations. This connection is observable through a unique NMR window.