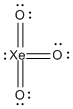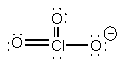CHM 501 Exam 1
Tuesday, Feb. 26, 2019
1. Predict the electron configuration for the following: a) Sc; b) Sc+; c) Sc3+; d) Sc3+.
Find all of the Russell-Saunders term symbols for each of these ions.
Answer
a) Sc [Ar]4s23d1; there is only one term – 2D
b) Sc+ [Ar]3d2
To find the terms: L = 4, and S = 1; the number of microstates = 10!/2!8! = 45
L\S |
1 |
0 |
etc. |
4 |
|
(2+,2–) |
|
3 |
(2+,1+) |
(2+,1–)
(2–,1+) |
|
2 |
(2+,0+) |
(2+,0–)
(2–,0+)
(1+,1–) |
|
1 |
(2+,–1+)
(1+,0+) |
(2+,–1–)
(2–,–1+)
(1+,0–)
(1–,0+) |
|
0 |
(2+,–2+)
(1+,–1+) |
(2+,–2–)
(2–,–2+)
(1+,–1–)
(1–,–1+)
(0+,0–) |
|
etc. |
|
|
|
L = 3, S = 1 gives 3F accounting for 21 microstates.
L = 1, S = 1 gives 3P accounting for 9 microstates.
L = 4, S = 0 gives 1G accounting for 9 microstates.
L = 2, S = 0 gives 1D accounting for 5 microstates.
L = 0, S = 0 gives 1S accounting for 1 microstates.
c) Sc2+ [Ar]3d1; there is only one term – 2D
d) Sc3+ [Ar]; there is only one term – 1S
2. Draw the lowest energy Lewis dot structure for each of the following, indicate the molecular geometry including an
estimate of all bond angles, and give the point group: a) AsF6–; b) XeO3;
c) SO3; d) ClO3–.
Answer
a) AsF6–
Lewis structure: 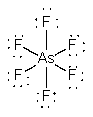
Geometry: octahedral
Bond angles: 90° and 180°
Point group: Oh
b) XeO3
Lewis structure: 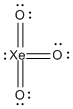
Geometry: pyramidal
Bond angles: 108° to 109°
Point group: C3v
a) SO3
Lewis structure: 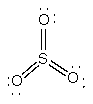
Geometry: trigonal planar
Bond angles: 120°
Point group: D3h
a) ClO3–
Lewis structure: 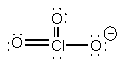
Geometry: pyramidal
Bond angles: 107° to 108°
Point group: C3v
3. Find the irreducible representations for the sigma bonds and lone pairs in PI3. What hybrid must be used?
Answer
PI3 belongs to the C3v point group.
C3v |
E |
2C3 |
3σv |
σ bonds |
3 |
0 |
1 |
lone pair |
1 |
1 |
1 |
The σ bonds transform as a1 + e
The lone pair transforms as a1
The hybrid on the P is sp3 (s is a1, pz is a1, and the
(px, py) pair are e)
4. Predict the relative solubility of NaCl in cyclohexanol, cyclohexane, and benzene. Indicate the weak interactions that
would promote the solubility. Would your prediction change for CsCl? Why or why not?
Answer
For NaCl the predicted solubility is cyclohexanol > benzene > cyclohexane. Cyclohexanol will have ion dipole interactions,
which will be the strongest. Cyclohexane and benzene both will have only van der Waal's and ion-induced dipole interactions but benzene
is more polarizable so will provide a more favorable interaction.
The prediction for CsCl would be similar. The only change will be that the Cs+ will be more polarizable.