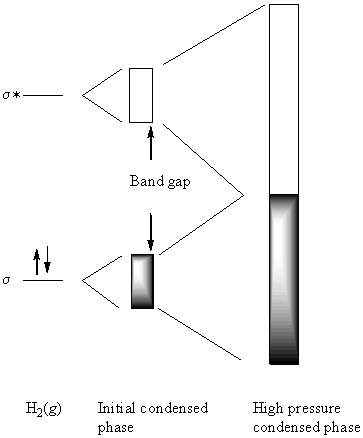
Molecular H2 has a filled σ orbital and unfilled σ* orbital. Upon condensation, these orbitals overlap with each other to form bands. At low pressure the energy difference between the bands is large so the hydrogen is an insulator. As the pressure is increased, the atoms get closer together, thereby increasing the amount of overlap between orbitals. This increases the bandwidth of each band. Eventually, the bottom of the σ* band has the same energy as the top of the σ band, leading to one large, partially filled band. This is the prescription for a metal. At pressures just before the band edges merge, the band gap will be small and the hydrogen could behave as a semiconductor.
