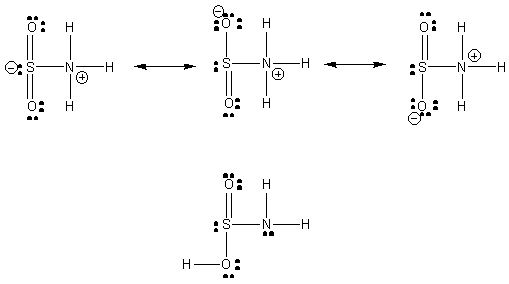

As in the SO3–NH3, the anticipated structure is
zwitterionic because of resonance stabilization. However, since the negative
charge is only spread out over 2 O atoms, the charge-neutral structure
may be quite close in energy. The two resonance structures to the right
should be a little lower in energy since the more electronegative O atom
bears the negative charge. Both the S and N centers should have approximately
109o bond angles, but the O-S-O and O-S-N angles should be reduced
a couple of degrees because of the lone-pair/bond-pair repulsion.