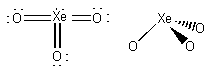
formal charges: O, 0; Xe, 0.
oxidation numbers: O, -2; Xe, +6.
geometric structure: pyramidal, O-Xe-O angles ~108o
Xe hybridization: sp3
point group: C3v
1. For each of the following species write the lowest energy
Lewis structure, indicate the formal charge and oxidation number of each
atom, show the geometric structure and estimate the bond angles, indicate
the σ hybridization about the central atom,
and give the point group. a) XeO3, b) XeOF2, c) XeO64-,
d) KrF2, e) SeF4.
a)
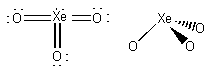
formal charges: O, 0; Xe, 0.
oxidation numbers: O, -2; Xe, +6.
geometric structure: pyramidal, O-Xe-O angles
~108o
Xe hybridization: sp3
point group: C3v
b)
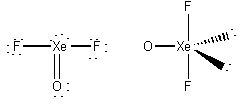
formal charges: O, 0; Xe, 0; F, 0.
oxidation numbers: O, -2; Xe, +4; F, -1.
geometric structure: bent-T, O-Xe-F angles ~89o
Xe hybridization: dsp3
point group: C2v
c)
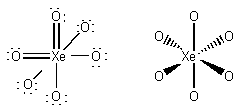
formal charges: =O, 0; -O, -1; Xe, 0.
oxidation numbers: O, -2; Xe, +8.
geometric structure: octahedral, all O-Xe-O angles
90o
Xe hybridization: d2sp3
point group: Oh
d)
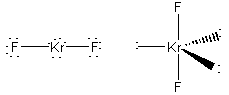
formal charges: F, 0; Kr, 0.
oxidation numbers: F, -1; Kr, +2.
geometric structure: linear, F-Kr-F 180o
Kr hybridization: dsp3
point group: D∞h
e)
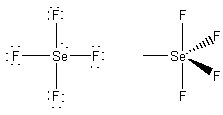
formal charges: F, 0; Se, 0.
oxidation numbers: F, -1; Se, +4.
geometric structure: see-saw, F-Se-F angles of
~118o and ~88o
Se hybridization: dsp3
point group: C2v