Are VBT and weak interactions adequate for description of the chemical and physical properties in simple molecules?
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
VBT helps explain everything except the paramagnetism of oxygen - this requires a different model.
Linear Combination of Atomic Orbitals LCAO
Basic assumptions:
1) Orbitals in molecules look a lot like atomic orbitals
2) Perturbations are caused by wave interference (overlap) of atomic orbitals;
3) The new molecular orbitals fill with electrons according to the Aufbau and Pauli Principles
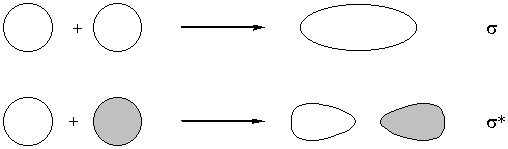
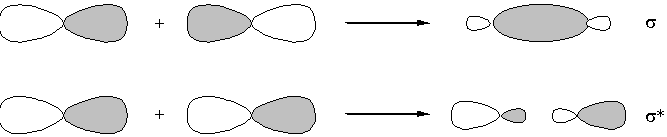
Constructive interference - lowers energy
Destructive interference - raises energy
Only orbital of the same irreducible representation can overlap with each other.
Increase of electron density in bonding orbitals between nuclei has two effects:
1) Screening of nuclear-nuclear repulsion by the extra electrons between nuclei
2) Electron-nuclear attraction in the direction that moves nuclei toward each other
Consider mixing orbitals in a linear diatomic molecule (D∞h)
s orbitals: a1g

p orbitals:
pz a1u
(px, py) e1u
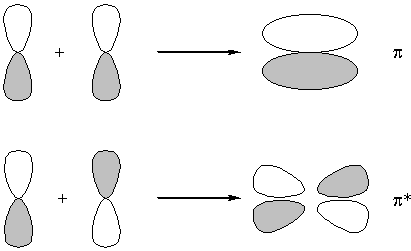
To distinguish various types of molecular orbitals
No node perpendicular to the atom-atom axis : bonding
One node perpendicular to the atom-atom axis : antibonding
No nodes parallel to the atom-atom axis : sigma
1 node parallel to the atom-atom axis : pi
2 nodes parallel to the atom-atom axis : delta
Basic principles
1. Choose atomic orbitals as basis set; #AOs initially = #MOs created
2. AOs of like size and energy overlap better with each other
3. Only orbitals of the same symmetry can overlap with each other
4. A larger overlap leads to a larger energy change
5. Fill electrons into orbitals following the Pauli and Aufbau principles
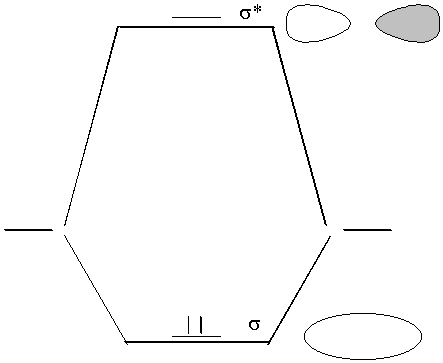
electron configuration σ2
BO = bond order = ½(Nb-Na)
where
Na = number of electrons in antibonding orbitals;
Nb = number of electrons in bonding orbitals
BO = 1 (in agreement with VBT)
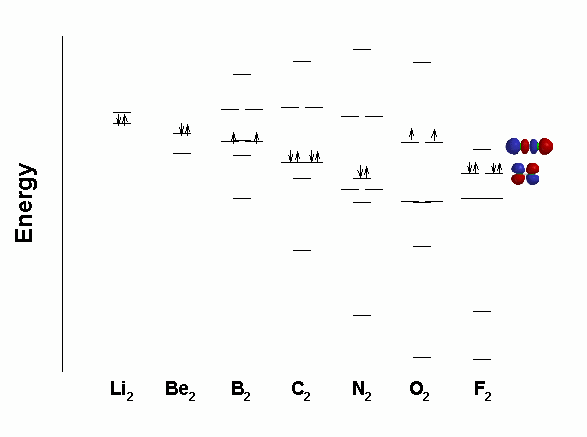
Note that 1σ* and 2σ are close in energy and of the same symmetry (σ) thus they can overlap (called a configuration interaction); overlap causes an energy change - an avoided crossing that changes the order of the orbitals:
| X2 |
|
|
|
|
| Li2 | 1σ2 |
|
|
|
| Be2 | 1σ21σ*2 |
|
|
|
| B2 | 1σ21σ*22π2 |
|
|
|
| C2 | 1σ21σ*2π4 |
|
|
|
| N2 | 1σ21σ*22σ2π4 |
|
|
|
| O2 | 1σ21σ*22σ2π4π*2 |
|
|
|
| F2 | 1σ21σ*22σ2π4π*4 |
|
|
|
| Ne2 | 1σ21σ*22σ2π4π*42σ*2 |
|
|
|
Neither B2 nor C2 has σ bond, only π bonds so the bond energies are quite low
CO as an example (very important molecule in inorganic chemistry; it has the strongest homonuclear bond energy and is a very good Lewis base towards low valent metals)
basis set is still 2s, 2p but now the orbital energies are different
Hybridization to form sp hybrids gives required lone pairs and then form molecular orbitals
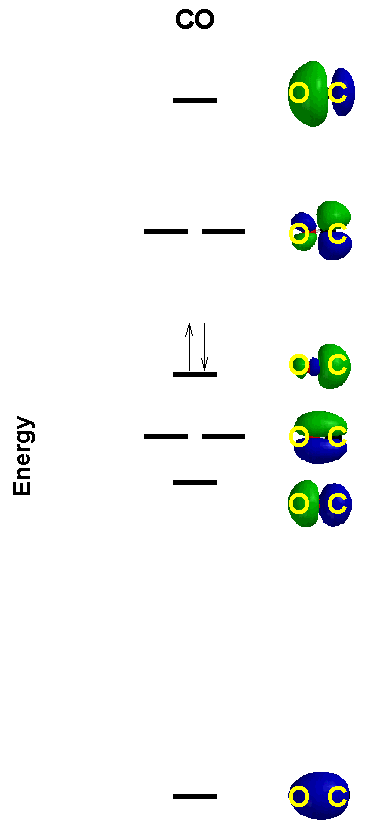
σ21nb2π42nb2
BO = 3
valence orbital is a lone pair on C so the basic end is C