Covalent bonding can be thought of as the situation when groups of atoms stay associated with each other independent of the phase
Valence Bond Theory: bonding occurs when pairs of electrons accumulate between nuclei
Why?

Paired electrons screen the nuclear charge, so nuclear-electron attraction overcomes electron-electron repulsion giving a net attraction of nuclei.
Electrons are distributed about atoms pictorially.
Only use valence electrons.
Lines represent an electron pair, either as a bond or a lone pair.
The formal charge about each atom should be minimized (neutral is best).
The octet "rule" works well for C, N, O, F but otherwise is merely advisory.
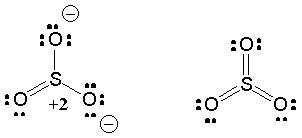
Resonance is a superposition of degenerate (equivalent) VB structures - this helps minimize charge distribution.
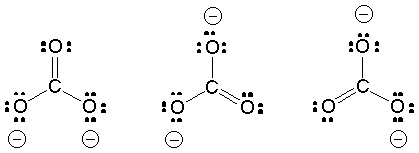
The net structure is a weighted average of all possible dot structures; the weighting is based on the relative energy of each resonance structure: low-energy structures contribute a lot and high-energy structures contribute little.
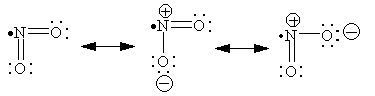
Neutral structure perhaps contributes less than ionic structures (Why?)
A simple application of Coulomb's Law to Lewis Dot structures - electron pairs distribute themselves to minimize electron-electron repulsion
Rules:
1) Draw the best Lewis Dot structure with minimum formal charges
2) Count the number of lone pairs and bonds (sb=db=tb) about the center of interest
3) Distribute the electron density as follows:
Count Distribution Geometry 2 linear 180o angles 3 trigonal planar
120o angles4 tetrahedral 109o angles 5 trigonal bipyramid 120o and
90o angles6 octahedral 90o angles 7 pentagonal bipyramid 72o and
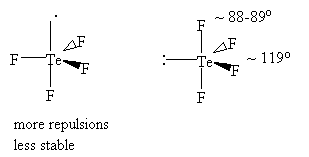
90o angles4) Distribute the lone pairs and bonds to minimize repulsions using the following:
lp-lp > lp-bp > bp-bp (tb > db > sb)
5) Fine geometry is dictated by lp-bp repulsions
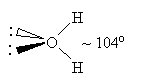
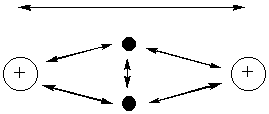
one way to "generate" molecular orbitals to be used in VB theory
Wave interference can occur on-site (same atom) as well as between atoms: this costs energy but the energy is recovered upon bond formation.
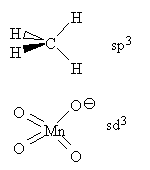
Hybridization probably occurs because of atom-atom repulsions so is more prevalent among first row atoms than anywhere else on the Periodic Table.
Understanding the relative phases of orbitals is important because constructive interference (orbitals with the same phase) increases orbital size while destructive interference (orbitals of opposite phase) decreases the orbital size.
Some common types of hybrids:
(convention : the unique axis is defined as the z axis)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|