CO insertion: 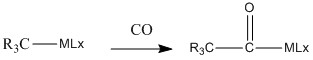
C2H4 insertion: 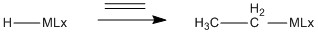
The catalyst and the reaction being catalyzed are in different phases. Normally the catalyst is a solid and the reaction is run in the solution or gas phase.
Heterogeneous catalysis has the advantage of not having to run a purification step to remove the catalyst from the product mixture. The disadvantage is that the surface sites used to cause the catalysis can be blocked or poisoned.
The classic example of heterogeneous catalysis is the Haber process for the reduction of N2 by H2 to give ammonia.
N2(g) + 3 H2(g) ⇄ Fe 2 NH3(g)
Industrially, this reaction is run at 450 °C and 100 atm
The equilibrium constant favors the products at room temperature but, in the absence of a catalyst, the activation energy is exceptionally high because of the extremely stable N2 triple bond.
Adsorption of the gases onto the iron surface promotes breaking of the diatomic bonds, increasing the rate.
Zeolites are aluminosilicates that are often used as solid acid catalysts, especially in cracking of crude oil.
Typical zeolites are Na2Al2Si3O10·2H2O (natrolite), or NaAlSi2O6·H2O (analcime).
Tee zeolites for interlocking AlO45– and SiO44– units that form large (molecular sized) pores.
The use of porous materials as catalysts has led to an extensive amount of research in Metal-Organic-Frameworks (MOFs).
MOFs are typically formed by using an ambidentate ligand that cannot chelate to crosslink metal ions to form crosslinked polymers. By changing the size of the linkers the pore size can be controlled. Dicarboxyllic acids are often used as the brdge.
Homogeneous catalysts are ones that are in the same phase as the reactants, typically in solution.
Catalysis by transition metal complexes is usually accomplished by some combination of commonly identifiable reactions: insertion, oxidative addition, and reductive elimination.
Insertion Reactions
In an insertion reaction a reactant inserts into a metal ligand bond, creating new bonds to the metal and the ligand.
Insertion reactions are most common in M-C and M-H bonds
Examples
CO insertion:
C2H4 insertion:
Oxidative Addition Reactions
In oxidative addition a reactant adds to an open coordination site while concurrently increasing the formal oxidation state of the metal. The metal is typically initially in low oxidation state. Four coordinate complexes are typically used in oxidative addition reactions. The reactant that is added may or may not have a bond broken
Examples
H2 addition:
CH3I addition:
O2 addition:
Reductive Elimination Reactions
Reductive elimination is the reverse of oxidative addition: the metal formal oxidation state is reduced and the coordination number of the metal is decreased. The eliminated product will have a new bond formed, so the eliminated groups (in most cases)) must be on adjacent (cis) coordination sites on the metal.
Examples
CH3CH3 elimination:
C6H5Cl elimination:
Examples of catalytic cycles
Hydrogenation using Wilkinson's Catalyst
[Rh(PPh3)3Cl] + H2 → cis-[Rh(PPh3)3ClH2] 1 oxidative Addition: Rh1+, 16 e– complex → Rh3+, 18 e– complex; 4- to 6-coordinate
cis-[Rh(PPh3)3ClH2] → cis-[Rh(PPh3)2ClH2] + PPh3 2 ligand elimination, 18 e– complex to 16 e– complex
cis-[Rh(PPh3)2ClH2] + R-CH=CH2 → cis-[Rh(PPh3)2ClH2(η2-R-CH=CH2)] 3 ligand addition (2 + 3 constitute a dissociative substitution reaction), 16 e– complex to 18 e– complex
cis-[Rh(PPh3)2ClH2(η2R-CH=CH2)] → cis-[Rh(PPh3)2ClH2(η1-R-CH-CH3)] 4 1,2-hydride shift, 18 e– complex to 16 e– complex
cis-[Rh(PPh3)2ClH2(η1-R-CH-CH3)] + PPh3 → fac-[Rh(PPh3)3ClH2(η1-R-CH-CH3)] 5 ligand addition, 16 e– complex to 18 e– complex
fac-[Rh(PPh3)3HCl(η1-R-CH-CH3)] → [Rh(PPh3)3Cl] + RCH2CH3 6 reductive elimination, 18 e– complex to 16 e– complex
Hydroformylation: RCH=CH2 + CO + H2 → RCH2CH2CHO
[Co(CO)4H] → [Co(CO)3H] + CO
[Co(CO)3H] + R-CH=CH2 → [Co(CO)3H(η2-R-CH=CH2
[Co(CO)3H(η2-R-CH=CH2 + CO → [Co(CO)4(η1CH2CH2R)]
[Co(CO)4(η1-CH2CH2R)] + CO → [Co(CO)4(η1-(C=O)CH2CH2R)]
[Co(CO)4(η1-(C=O)CH2CH2R)] + H2 → [Co(CO)4H] + RCH2CH2CHO
Alkene Metathesis
In general, alkene metathesis is an exchange of substituent groups between two different alkenes:
Grubb's and Shrock won a Nobel Prize for creating catalysts for this reaction.
An interesting use for this is Ring Opening Metathesis Polymerization (ROMP):