Fe d8 (Note: when in a bonding situation, all of the electrons on a metal move to the orbital with the lowest n quantum number, similar to when ionization happens.)
CO 5(2) = 10
8 + 10 = 18
An organometallic complex is defined as a molecule or ion with a metal to carbon bond.
Organometallic complexes occur predominantly with low oxidation state transition metals.
The bonding in these complexes is usually best interpreted using covalent theories.
Effective Atomic Number Rule (EAN) (a VBT approach): complexes with 18 (sometimes 16) electrons are stable (analogous to octet rule for main group elements)
Count the metal valence electrons and the electrons donated by the ligands; if this adds up to 18 (or 16), then the complex is predicted to be stable
Unlike the main group, d orbital lone pairs are generally not stereochemically active so the geometries of these compounds normally are determined solely by the number of ligands.
Fe(CO)5
Fe d8 (Note: when in a bonding situation, all of the electrons on a metal move to the orbital with the lowest n quantum number, similar to when ionization happens.)
CO 5(2) = 10
8 + 10 = 18
Fe(cp)2
Fe2+ d6
cp– 2(6) = 12
6 + 12 = 18
Co(cp)2
Co2+ d7
cp– 2(6) = 12
7 + 12 = 19 not stable but Co(cp)2+ is stable
Mn2(CO)10
on each Mn: Mn d7
CO 5(2) = 10
M-M bond, 1
7 + 10 + 1 = 18
[PtCl3(CH2=CH2)]–
Pt2+ d8
Cl– 3(2) = 6
C2H4, 2
8 + 6 + 2 = 16
Molecular Orbital approach in an Oh complex:
Group Theory tells us how the ligand and metal orbitals overlap. Since only orbitals of the same irreducible representation overlap to form molecular orbitals, identifying the irreducible representations aids in the determination of the molecular orbitals.
The irreducible representations for the d orbitals on the metal are easily found from the point group:
s orbitals: a1g
p orbitals: tiu
d orbitals: t2g (dxy, dyz,dxz) and eg (dx2–y2, dz2)
The ligand orbitals total representation must be found using the symmetry operations.
For the ligand sigma orbitals, the irreducible representations transform as a1g + eg + t1u
Using projection operators the ligand group orbitals look like:
a1g: Ψ(a1g) = (σ1 + σ2 + σ3 + σ4 + σ5 + σ6)/√6
eg: Ψ1(eg) = (2σ1 – σ2 – σ3 – σ4 – σ5 + 2σ6)/√12 and Ψ2(eg) = (σ2 – σ3 + σ4 – σ5)/2
t1u: Ψ1(t1u) = (σ1 – σ6)/√2, Ψ2(t1u) = (σ2 – σ4)/√2, Ψ3(t1u) = (σ3 – σ5)/√2
Graphically:
The MO diagram then can be drawn as:
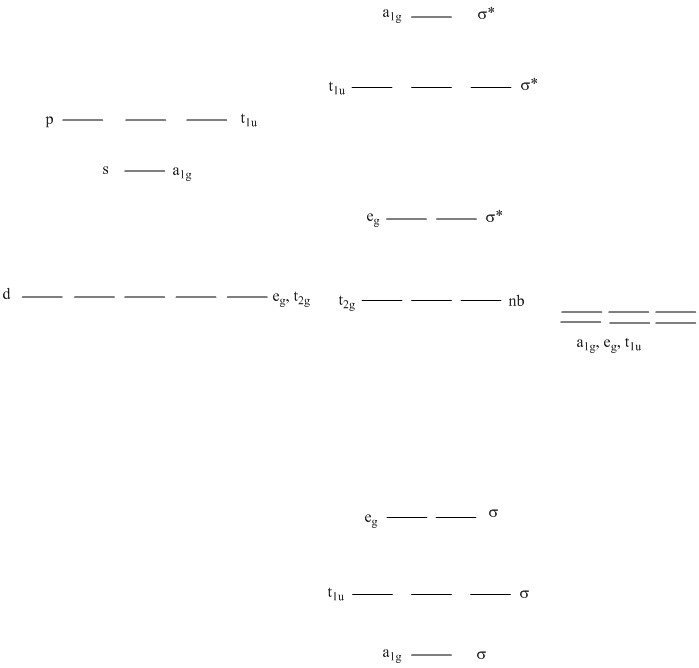
The nb and lowest energy σ* orbitals are similar to the Crystal Field Theory description. The nb orbitals are strictly d like (dxy, dyz, dxz) and the σ* are mostly d orbital like (dx2–y2, dz2).
Inclusion of π bonding means using the t2g orbitals for overlap:
If the t2g orbitals are empty, then the ligand needs filled π orbitals to create a M-L π bond; the t2g orbitals become somewhat antibonding, are destabilized, and 10Dq is reduced.
If the t2g orbitals are occupied, then the ligand needs unfilled π* orbitals to create the M-L π bond; the t2g orbitals become somewhat bonding, are stabilized, and 10 Dq is increased.
This is known as π backbonding. It is the reason CO is such a strong ligand.
CO
CO can act as a terminal or bridging ligand. It is a strong sigma donor and a good pi acceptor. It is a good ligand for bonding studies because it has a strong IR absorption (in complexes) in a region of the spectrum where there are few other functional groups that absorb.
Fe(CO)5: D3h, liquid at room temperature, toxic, decomposes easily, follows the 18 electron rule, has a single NMR absorption in the 13C spectrum indicating rapid exchange of the ligands between the axial and equatorial positions.
Fe2(CO)9
Does not follow 18 electron rule, has two IR peaks in the CO region, current understanding is that there is no Fe-Fe bond.
Co(CO)5: not stable (19 electron system), easily oxidized to [Co(CO)5]+
Co2(CO)8
Stable by the EAN rule, includes a metal-metal bond
cp: ηx-cyclopentadienide (and the related Me5cp)
cp bonds through the π system; can bond through any number of carbons; the ligand acts as an aromatic system
Ti(cp)4 is [Ti(η1-cp)2(η5-cp)2]
This makes it a 16 electron complex, which is stable.
There are bis-cp complexes of most of the transition metals
alkenes and alkynes
Can be either η1 or η2
[Rh2Cl2(CH2=CH2)4]
cyclooctadiene (cod) is often used in syntheses because it is a good leaving group:
Ni(cod)2:
carbenes: R2C:
There has been a lot of recent interest in carbene chemistry
[VOCl3(C3H4N2(mes)2)]
The carbene stabilizes the +5 oxidation state while still being an organometallic complex.