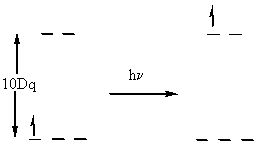
hν = 10Dq in this case
The number of relatively low lying energy states associated with transition metal complexes means that the absorption of light is useful and informative. The accessible excited states also introduce the possibility of interesting photochemistry.
Unfortunately, transitions from d orbitals to d orbitals are formally forbidden (the selection rule is that Δl = ±1 to be allowed; for d → d transitions Δl = 0. This is known as LaPorte forbidden). Physically, there must be a change in the dipole moment for the light to be absorbed. In addition, allowed transitions must conserve the spin degeneracy. That is, the ground and excited states must have the same spin degeneracy.
More rigorously for electronic states in complexes the transition dipole moment (the intensity of the transition is the square of this) is given by ∫Ψ*μΨdτ where Ψ* is the excited state wavefunction, Ψ is the ground state wavefunction, μ is the electric dipole operator, and the integration is done over all space.
This is a difficult integral to evaluate but we can determine if the value is zero or nonzero using group theory.
If the value is zero, we know that the transition is forbidden.
Group theory tells us that for the integral to be nonzero (i.e., an allowed transition) the cross product of the irreducible representations for each term in the integral must contain the totally symmetric representation. If group theory indicates an allowed transition, the size of that transition moment is not given by group theory.
Thus: Rep(Ψ*)×Rep(μ)×Rep(Ψ) must give the totally symmetric rep (plus others) to be allowed.
The reducible rep for the cross product is found simply by multiplying the characters in the character table for each symmetry element.
μ = –er (e is the elementary charge) and r is the cartesion coordinate, i.e., x, y, or z. The reps for x, y, and z can be found directly from the character table.
The reps for the wavefunctions are found using the techniques we discussed earlier.
A simple example: d1
The ground state is 2T2g and the excited state is 2Eg and the x, y, and z coordinates transform as a degenerate group, T1u
The cross product then is
Oh
E
8C3
6C2
6C4
3C2 (=C42)
i
6S4
8S6
3σh
6σd
Eg
2
–1
0
0
2
2
0
–1
2
0
T2g
3
0
1
–1
–1
3
–1
0
–1
1
T1u
3
0
–1
1
–1
–3
–1
0
1
1
total rep
18
0
0
0
2
–18
0
0
–2
0
This transforms as A1u + A2u + 2Eu + 2T1u + 2T2u
Since the A1g representation is not present, the transition is not allowed.
Experimentally, we can observe d–d transitions. Why?
The answer is vibrations: Coupling the electronic state to a vibrational state can make absorption allowed. From a group theoretical perspective, that means adding a rep for a vibration to the cross product. For example, in the above example, if an A1u vibrational state can couple to the ground state, the cross product will now include the A1g irreducible representation so that the transition becomes formally allowed (although weak).
Measurement of Dq is usually done spectroscopically, move electron from t2g to eg orbital with no spin change
hν = 10Dq in this case
Because of electron–electron repulsion, the lowest energy transition is not always equal to 10Dq. In the weak field limit the transition energies are given approximately by:
ConfigurationLowest Energy Spin Allowed Transition (Oh)
d110Dq
d28Dq
d310Dq
d4 (hs)10Dq
d4 (ls)~9Dq
d5 (hs)none allowed
d5 (ls)~8.5Dq
d6 (hs)10Dq
d6 (ls)~9Dq
d7 (hs)8Dq
d7 (ls)~9Dq
d810Dq
d910Dq
Complication : charge transfer transitions
M–L
M+L– Metal to Ligand Charge Transfer (MLCT)
M–L
M–L+ Ligand to Metal Charge Transfer (LMCT)
CT transitions are usually much more intense than d–d transitions so can be distinguished by molar absorptivity
ε (CT) ~ 103 – 104 L/mol–cm
ε (d–d spin allowed) ~ 101 – 102 L/mol–cm
ε (d–d spin forbidden) ~ 10–1 – 100 L/mol–cm
Exact formulas for some cases have been published: A. B. P. Lever, J. Chem. Ed., 1968, 45, 711 – 712; Y.-S. Dou, J. Chem. Ed., 1990, 67, 134.
For d2 and d7(hs) Oh complexes three spin allowed transitions are predicted at energies given by
ν1 = –7.5B + 5Dq + 0.5(225B2 + 100Dq2 + 180BDq)½ 3T2g ← 3T1g (d2) or 4T2g ← 4T1g (d7)
ν2 = –7.5B + 15Dq + 0.5(225B2 + 100Dq2 + 180BDq)½ 3A2g ← 3T1g (d2) or 4A2g ← 4T1g (d7)
ν3 = (225B2 + 100Dq2 + 180BDq)½ 3T1g(P) ← 3T1g (d2) or 4T1g(P) ← 4T1g (d7)
If all three transitions are observed, then 10Dq = ν2 – ν1 and 15B = ν3 + ν2 – 3ν1
If ν3 is obscured by a CT band, then B can be found by 3B = [(ν2 – 2ν1)ν1]/[9ν1 – 4ν2]
Likewise, for d3 and d8 Oh complexes three spin allowed transitions are predicted at energies given by
ν1 = 10Dq4T2g ← 4A2g (d3) or 3T2g ← 3A1g (d8)
ν2 = 7.5B + 15Dq – 0.5(225B2 + 100Dq2 – 180BDq)½ 4T1g(F) ← 4A2g (d3) or 3T1g(F) ← 3A2g (d8)
ν3 = 7.5B + 15Dq + 0.5(225B2 + 100Dq2 – 180BDq)½ 4T1g(P) ← 4A2g (d3) or 3T1g(P) ← 3A2g (d8)
If all three transitions are observed, then B can easily found from 15B = ν3 + ν2 – 3ν1
If ν3 is obscured by a CT band, which is often the case, then B can be found by 3B = [(ν2 – 2ν1)(ν2 – ν1)]/[5ν2 – 9ν1]
For d6(ls) the following formulas can be used (D. R. Brown, R. R. Pavlis, J. Chem. Ed., 1985, 62, 807 – 808).
ν1 = 10Dq – 4B + 86B2/10Dq 1T1g ← 1A1g
ν2 = 10Dq + 12B + 2B2/10Dq 1T2g ← 1A1g
To use these formulas requires solving two quadratic equations in two unknowns.
When simple formulas don't work or there are spin-forbidden transitions observed, then Tanabe-Sugano diagrams are used
d2
d3
d4
d5
d6
d7
d8
An example. The spectrum of [Cr(NH3)6]3+ is shown below. Find 10q and B.
This is a d3 complex. There are two approaches to finding B and 10Dq - use formulas and to use the Tanabe-Sugano diagram.
There are two spin-allowed transitions shown (log(ε) a little less than 2) at 463 nm and 351 nm.
In units of energy (cm–1) these are ν1 = 21600 cm–1 and ν2 = 28500 cm–1
Using the closed formulas ν1 = 10Dq = 21600 cm–1 for 4T2g ← 4A2g
Since only two peaks are available, B is found using 3B = [(ν2 – 2ν1)(ν2 – ν1)]/[5ν2 – 9ν1] = [(28500) – 2(21600)][(28500) – (21600)]/[5(28500) – 9(21600)] = 1950 cm–1
B = 650 cm–1
Using the Tanabe-Sugano diagram: ν2/ν1 = 28500/21600 = 1.32
A ratio of ν2/ν1 = 1.31 (= 51/39) at Dq/B = 4
E/B = 39 gives B = E/39 = 21600/39 = 554 cm–1 and E/B = 51 gives B = E/51 = 28500/51 = 559 cm–1. The average B = 557 cm–1
Dq/B = 4 gives Dq = 4B = 4(557) = 2280 cm–1 so 10Dq = 22800 cm–1
The lowest energy spin-forbidden transition (22Eg ← 4A2g and 2T1g ← 4A2g, which will be at about the same energy) is at E/B ~ 22 so E = 22B = 12300 cm–1 or ν = 813 nm
The next lowest spin-forbidden transition is 2T2g ← 4A2g at E/B ~ 34 so E = 34B = 18900 cm–1 or ν = 529 nm
Neither prediction for the spin-forbidden transition matches the observation at ~650 nm, suggesting that a different C/B ratio is required.
The closed formula method gives better results because it removes the ambiguity of reading a graph. Thus, 10Dq = 21600 cm–1 and B = 650 cm–1, implying a nephelauxetic parameter of β = 650/1030 = 0.63.
Because spin and orbits are angular momentum functions, there can be a coupling between these two quantities, known as spin-orbit coupling.
Quantitatively, this is written as λL·S where L and S are the orbital angular and spin angular momentum operators, respectively.
For free ions this is fairly easy to evaluate:
J = L + S is the total angular momentum
J2 = (L + S)2
J2 = L2 + S2 + 2L·S
For any angular momentum operator, X, evaluation of the operator squared is X2 = X(X+1), where X is the quantum number associated with the operator. Thus,
J(J+1) = L(L+1) + S(S+1) + 2L·S
Thus, λL·S = ½λ[J(J+1) – L(L+1) – S(S+1)]
The consequence of spin-orbit coupling is that ground states mix, to a small degree, with excited states of different spin multiplicity. This allows weak transitions that are formally spin-forbidden.