A better treatment of the bonding in transition metal complexes needs to add a quantum mechanics component and to treat electronic states rather than just a one-electron approach.
Starting with the atomic d orbitals, we know that they split into a number of states simply based on the occupation of the electrons in the different orbitals. We denote these using Term Symbols. The different terms have different energies because of e––e– repulsion.
The energies of the different states can be calculated using standard quantum mechanical techniques. In general, the energy of each Term can be expressed as a sum of integrals. Depending on how the linear combinations are expressed, these integrals are expressed as Condon-Shortley parameters (Fo, F2, F4) or Racah parameters (A, B, C). Inorganic chemists typically use Racah parameters. For each electron configuration and Term, there is a different linear combination A, B, and/or C that expresses the energy of that state.
From G. Racah, Phys. Rev., 1962, 62, 438.
electron configurationTermRepulsion Energy
d2, d8
3FA – 8B
3PA + 7B
1GA + 4B + 2C
1DA – 3B + 2C
1SA + 14B + 7C
d3, d7
4F3A – 15B
4P3A
2H3A – 6B + 3C
2G3A – 11B + 3C
2F3A + 9B + 3C
2D (2 states)3A + 5B + 5C ± (193B2 + 8BC + 4C2)½
2P3A – 6B + 3C
d4, d6
5D6A – 21B
3H6A – 17B + 4C
3G6A – 12B + 4C
3F (2 states)6A – 5B + 11C/2 ± (3/2)(68B2 + 4BC + C2)½
3P (2 states)6A – 5B + 11C/2 ± ½(912B2 – 24BC + 9C2)½
3D6A – 5B + 4C
1I6A – 15B + 6C
1G (2 states)6A – 5B + 15C/2 ± ½(708B2 – 12BC + 9C2)½
1F6A + 6C
1D (2 states)6A + 9B + 15C/2 ± (3/2)(144B2 + 8BC + C2)½
d5
6S10A – 35B
4G10A – 25B + 5C
4F10A – 13B + 7C
4D10A – 18B + 5C
4P10A – 28B + 7C
2I10A – 24B + 8C
2H10A – 22B + 10C
2G10A – 13B + 8C
2G'10A + 3B + 10C
2F10A – 9B + 8C
2F'10A – 25B + 10C
2D (2 states)10A – 3B + 11C ± 3(57B2 + 2BC + C2)½
2D'10A – 4B + 10C
2P10A + 20B + 10C
2S10A – 3B + 8C
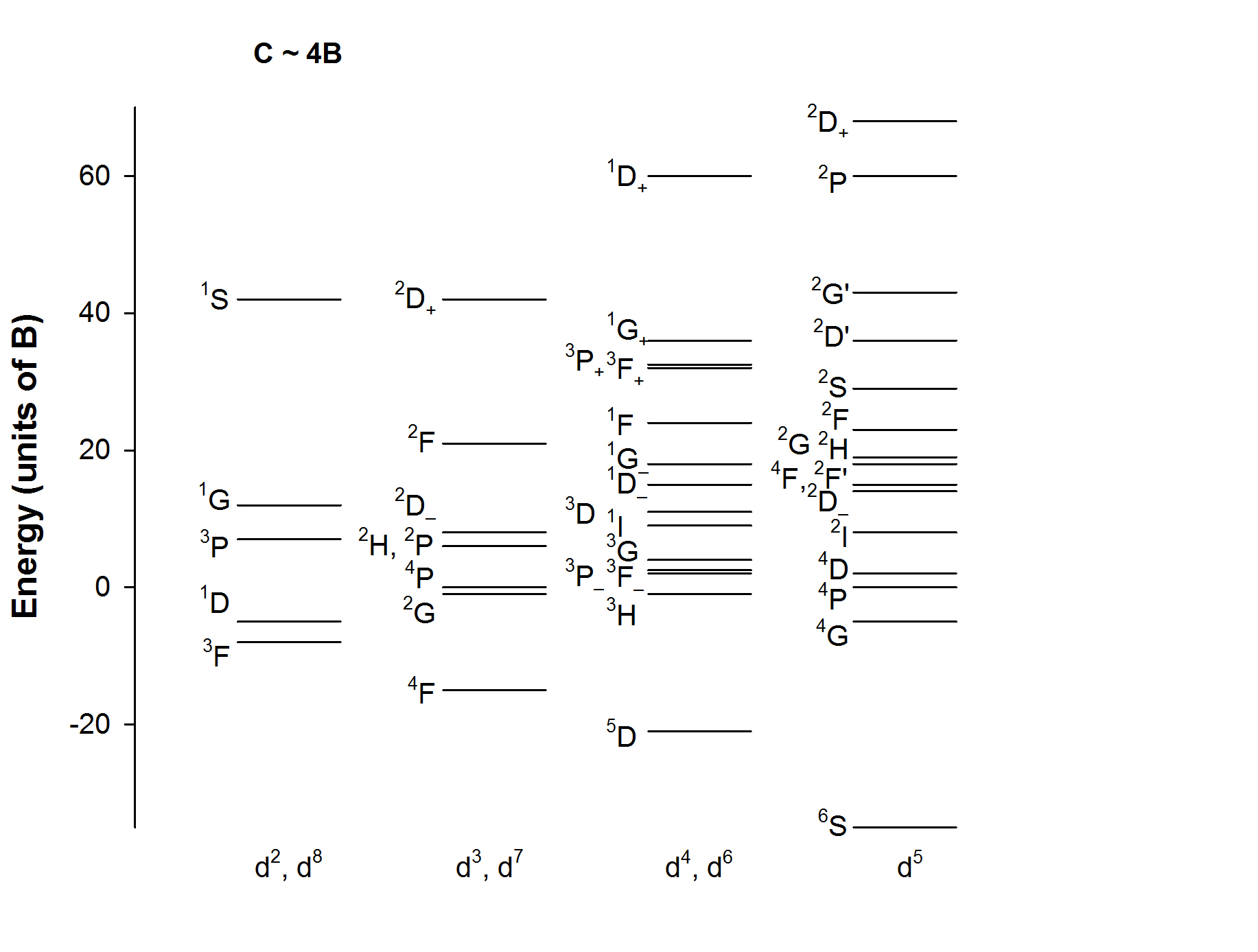
Note that for each electron configuration the repulsion associated with A is constant within all of the energy levels for that electron configuration. Consequently, this term is typically ignored or considered the zero of energy.
Typically, C ~ 4B. Under this assumption, the energy levels for each electron configuration are approximately shown below.

The B parameter varies depending on the ligand and can be measured in many cases. Like the spectrochemical series, the changes in B is largely independent of the metal ion and depends mostly on the bonding of the ligand. B is always reduced from the free ion value because some electron density is moved from the metal to the ligand, which reduces electron-electron repulsion. The reduction of B caused by bonding is called the nephelauxetic effect.
The nephelauxetic parameter is defined as β = B(complex)/B(free ion). The smaller β is the more covalency in the metal-ligand bond.
In the presence of an octahedral field, most of these states splits into two or more states, which can be determined and labeled using group theory in the Oh point group.
For each free electron state. the characters of the O point group (a subgroup of Oh known as a rotation group because the group only contains rotation symmetry elements) are found using the formula Χ(θ) = sin[(L + ½)θ]/sin[θ/2] where L = the orbital angular momentum quantum number associated with the state (L = 0 for S, L = 1 for P, etc.) and θ is the rotation angle for the symmetry angle, and θ ≠ 0. For the E symmetry element, Χ(E) = 2L + 1, the orbital degeneracy of the state.
In Oh the representation determined by the character of the i symmetry element for the type of orbital being considered, i.e. +1 for s, d,and g orbitals and –1 for p and f orbitals. For Χ(i) = +1, the subscript is "g" (gerade) and for Χ(i = –1 the subscript is "u" (ungerade).
Using this method, for the d orbitals the results are:
Free Electron StateState in Oh
SA1g
PT1g
DT2g + Eg
FA2g + T1g + T2g
GA1g + Eg + T1g + T2g
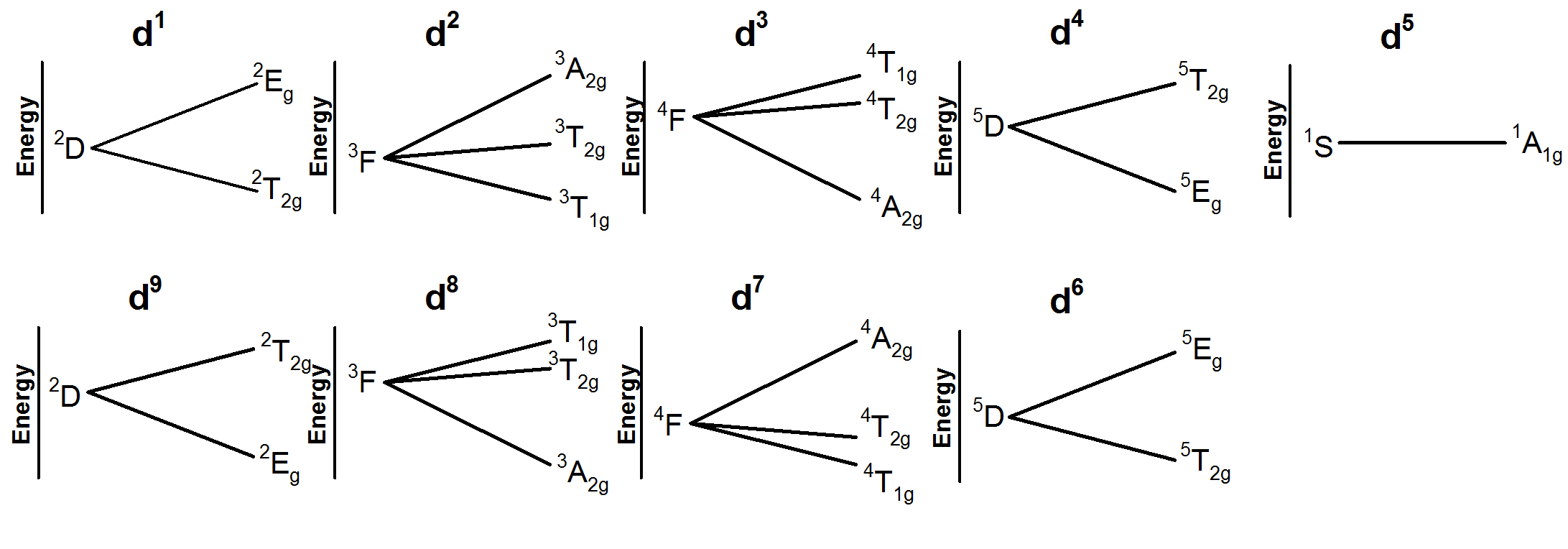
For the ground state free electron states, in a weak Oh field causes the energy level to split:
d1, d92D → 2Eg + 2T2g
d2, d83F → 3A2g + 3T1g + 3T2g
d3, d74F → 4A2g + 4T1g + 4T2g
d4, d65D → 5Eg + 5T2g
d56S → 6A1g
The splitting of the lowest energy levels in a weak field are shown below. When all of the same spin degeneracy states are included these are known as Orgel diagrams.
Similar splitting occurs in excited states, which can be labeled using group theory.
In a strong field the relative energies can be found by using the electron configurations.
d1E(t2g1) = –4Dq; E(eg1) = 6Dq
d2E(t2g2) = –8Dq; E(t2g1eg1) = 2Dq E(eg2) = 12Dq
d3E(t2g3) = –12Dq; E(t2g2eg1) = –2Dq E(t2g1eg2) = 8Dq E(eg3) = 18Dq
d4E(t2g4) = –16Dq; E(t2g3eg1) = –6Dq E(t2g2eg2) = 4Dq E(t2g1eg3) = 14Dq E(eg4) = 24Dq
d5E(t2g5) = –20Dq; E(t2g4eg1) = –10Dq E(t2g3eg2) = 0Dq E(t2g2eg3) = 10Dq E(t2g1eg4) = 20Dq
d6E(t2g6) = –24Dq; E(t2g5eg1) = –14Dq E(t2g4eg2) = –4Dq E(t2g3eg3) = 6Dq E(t2g2eg4) = 16Dq
d7E(t2g6eg1) = –18Dq E(t2g5eg2) = –8Dq E(t2g4eg3) = 2Dq E(t2g3eg4) = 12Dq
d8E(t2g6eg2) = –12Dq E(t2g5eg3) = –2Dq E(t2g4eg4) = 8Dq
d9E(t2g6eg3) = –6Dq E(t2g5eg4) = 4Dq
Further splitting of energy levels occurs when the symmetry is lower than Oh. Again, group theory allows us to label states using Correlation Tables.
Point Group
Oh
O
Td
D4h
D2d
C4v
C2v
D3d
D3
C2h
Irreducible Representation
a1g
a1
a1
a1g
a1
a1
a1
a1g
a1
ag
a2g
a2
a2
b1g
b1
b1
a2
a2g
a2
bg
eg
e
e
a1g + b1g
a1 + b1
a1 + b1
a1 + a2
eg
e
ag + bg
t1g
t1
t1
a2g + eg
a2 + e
a2 + e
a2 + b1 + b2
a2g + eg
a2 + e
ag + 2bg
t2g
t2
t2
b2g + eg
b2 + e
a2 + e
a1 + b1 + b2
a1g + eg
a1 + e
2ag + bg
a1u
a1
a2
a1u
b1
a2
a2
a1u
a1
au
a2u
a2
a1
b1u
a1
b2
a1
a2u
a2
bu
eu
e
e
a1u + b1u
a1 + b1
a2 + b2
a1 + a2
eu
e
au + bu
t1u
t1
t2
a2u + eu
b2 + e
a1 + e
a1 + b1 + b2
a2u + eu
a2 + e
au + 2bu
t2u
t2
t1
b2u + eu
a2 + e
b1 + e
a2 + b1 + b2
a1u + eu
a1 + e
2au + bu
Once the point group is identified, the splitting of the states can be written easily using the correlation table. The energies of the states can not be established using group theory.
A simple example: d1
The free ion is 2D that splits into 2T2g (ground state) and 2Eg states. Since the 2T2g state is triply degenerate, it must further split (Jahn-Teller effect) to remove the degeneracy. Lowering the symmetry to D4h (by compression of the ligands along the z-axis) the energy states become 2T2g → 2B2g (ground state) + 2Eg and 2Eg → 2A1g + 2B1g
Another example: What are the fourteen lowest energy states for [Cr(NH3)5Cl]2+
[Cr(NH3)5Cl]2+ is a d3 complex with C4v symmetry. Thus, the energy level diagram is: