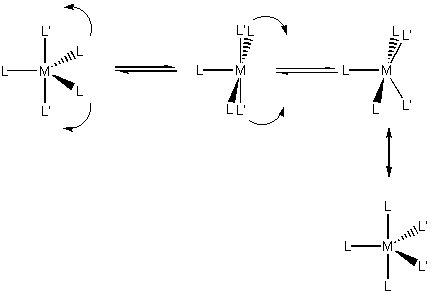
Two geometries-trigonal bipyramid (D3h) and square-based pyramid (C4v); the two geometries are generally close in energy so ligands can scramble.
Berry pseudorotation is seen in mixed ligand complexes, this scrambles ligand positions:
d block chemistry is vast, often thought of in terms of Lewis Acid/Base interactions
Metals act as Lewis acids, which react with Lewis bases
The Lewis acid/base product is generally called a coordination complex and the Lewis bases are called ligands
Wide variety of d-block chemistry to be addressed:
geometries: coordination number (CN = the number of Lewis bases bonded to the metal) can vary from 2 to 8; 4 and 6 are most common
oxidation states: from negative to +7 or +8
bonding: nearly purely ionic to nearly purely covalent; intermediate much more common
reactivity: substitution, addition, elimination, redox - and a number of mechanisms
Geometries range from tetrahedral (Td) to square planar (D4h) with some complexes are known that are between Td and D4h (D2d)
Square planar complexes can exhibit geometric isomerism: ML2L'2 cis or trans makes a difference in the chemistry: cis-Pt(NH3)2Cl2 is an anticancer drug, the trans isomer is inert
Two geometries-trigonal bipyramid (D3h) and square-based pyramid (C4v); the two geometries are generally close in energy so ligands can scramble.
Berry pseudorotation is seen in mixed ligand complexes, this scrambles ligand positions:
Most common coordination number, octahedral
Often can get distortions from Oh
Extension or compression along one axis : tetragonal : D4h
Change along two axes : rhombic : D2h
Change along the diagonal (the C3 axis) : D3d
Substitutions:
disubstituted ML4L'2 : cis (C2v) or trans (D4h)
trisubstituted ML3L'3 : fac (C3v) or mer (C2v)
Very rare for first row (steric), found occasionally in 2nd or 3rd row complexes
Usually ligands are Lewis bases (nonmetals, either anions or neutrals with lone pairs) but this is not required.
The ligand could be another metal complex to give a multimetal compound
Re2Cl82– (D4h)
(quadruple metal-metal bond)
Traditional ligands have N, O, halide, S, P as the binding site (all have lone pairs)
Binding of C to metals is also well known - this is organometallic chemistry
Ligands may have multiple binding sites:
monodentate : one binding site NH3, Cl–, H2O, CN–
ambidentate : two available binding sites but only one can be used CN–, SCN–.
This gives the possibility of linkage isomerization : M-SCN differs from M-NCS. To indicate which atom in an ambidentate ligand binds to the metal the notation used is ligand name-κbinding atom (κ = Greek letter kappa); for example for SCN– bound through the S atom the naming convention is thiocyanato-κS ...
bidentate : two binding sites and both used
ethylenediamine (en), oxalate (ox), 2,2'-bipyridine (bipy), acetylacetonate (acac), phenanthrene (phen)
chelation : ligand plus metal form a ring system; requires a multidentate ligand
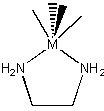
Chelation leads to the possibility of chirality in a coordination complex:
For example: [Ni(en)3]2+ has two possible enantiomers
The easiest way to determine if a molecule or ion is chiral or not is to identify the point group: if the point group does not contain a mirror nor an inversion, then the molecule or ion is chiral. If σ or i is present, then there is no chirality.
tridentate : three binding sites used
H2NCH2CH2NHCH2CH2NH2 (dien)
quadridentate : four binding sites
porphyrin
polydentate : many binding sites
EDTA4– (–O2CCH2)2–NCH2CH2N–(CH2CO2–)2
binds strongly to nearly all metals; used to treat heavy metal poisoning
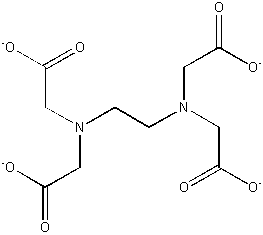
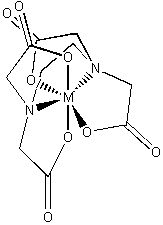
Simple coordination complexes are generally synthesized by adding ligands to a solvated metal ion, typically using water as a solvent:
[M(OH2)6]m+ + nL ⇄ [M(OH2)6–nLn]m+ + n H2O
The equilibrium constant for the reaction is Keq = βn = [[M(OH2)6–nLn]m+]/[[M(OH2)6]m+][L]n
The extent of reaction, i.e. the maximum value for m, depends on the ligand, solvent, and metal ion.
By using titration techniques, the stepwise equilibrium constants, called formation constants, can be evaluated:
[M(OH2)6]m+ + L ⇄ [M(OH2)5L]m+ + H2O Kf1 = [[M(OH2)5L]m+]/[[M(OH2)6]m+][L]
[M(OH2)5L]m+ + L ⇄ [M(OH2)4L2]m+ + H2O Kf2 = [[M(OH2)4L2]m+]/[[M(OH2)5L]m+][L]
[M(OH2)4L2]m+ + L ⇄ [M(OH2)3L3]m+ + H2O Kf3 = [[M(OH2)3L3]m+]/[[M(OH2)4L2]m+][L]
etc.
Then βn = Kf1Kf2...Kfn
Dissociation constants are the inverse of formation constants, i.e. Kd1 = 1/Kf1, etc.
Based on statistical considerations Kfn > Kfn+1
Deviations from this prediction indicates structural or electronic effects need to be considered.
Possible reasons:
Chelation
Macrocycles
Steric Repulsion
Change in Coordination Number
Electronic Delocalization (comparable to conjugation in organic chemistry)