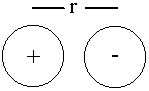
Properties: hard, brittle, high mp and bp, often water soluble, electrolytes in solution or melt, usually crystalline. All of this suggests large bond energies.
Necessary conditions for an ionic compound: cation (implies neutral species has a low ionization energy) and anion (implies neutral has a high electron affinity)
We develop a theory of ionic bonding using simple electrostatics. Assume that the ions behave as point charges (i.e., the ions have no volume). Then build a lattice from condensation of gas phase ions and use Coulomb's law to calculate the energy.
Ion Pair
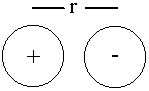
Edip = q+q–/4πεor
q+, q- are the ionic charges in coulombs
r is the distance between ions in meters
εo is the permittivity of free space
Ion square
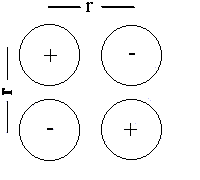
Esquare = 4q+q–/4πεor + q+q+/4πεo√2r + q–q–/4πεo√2r
Since (in this example) q+=–q–
Esquare = q+q–(4 – 1/√2 – 1/√2)/4πεor = 2.586Edip
ion cube
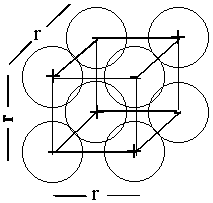
Ecube = 12q+q–/4πεor + 6q+q+/4πεo√2r + 6q–q–/4πεo√2r + 4q+q–/4πεo√3r
Ecube = q+q–(12 – 6/√2 – 6/√2 + 4/√3)/4πεor = 5.824Edip
Continue this over the entire lattice to give:
ECoul = N0MZ+Z–e2/4πεor
N0 = Avogadro's number
Z+, Z- are the ionic charges in units of e (i.e., +1, +2, -1, -2, etc.)
r is the interionic distance
M is called the Madelung constant.
M depends solely on geometry, i.e., the kind of lattice but the internuclear distances are factored out. Madelung constants are tabulated for all the common lattice types.
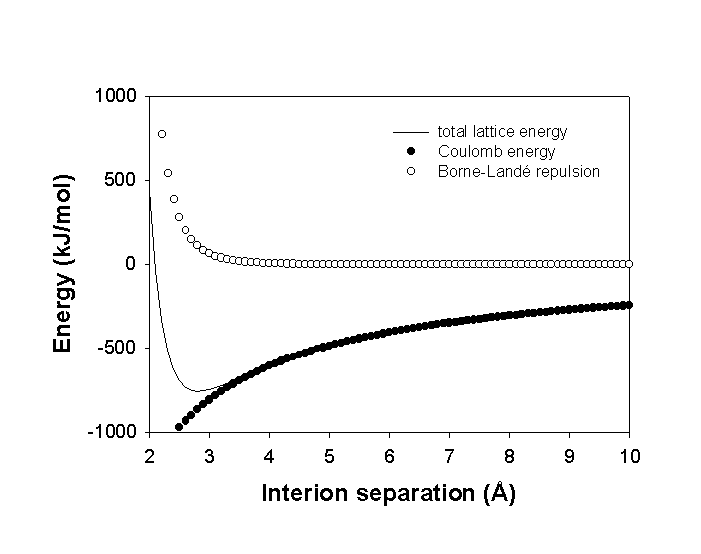
ECoul is negative (all terms are positive except Z–), i.e., the bonding is favorable. In fact, too favorable: the most stable situation is when r = 0.
Why? Initial point charge assumption does not allow for nuclear-nuclear repulsion at close distances.
Correct this empirically: add a term that is repulsive at small r but negligible at larger distances. This means either an exponential term or a 1/rn term with large n.
Born repulsion:
Erep = B/rn
n is known as the Born exponent, B is an arbitrary constant
The lattice energy becomes
Elat = ECoul + Erep = N0MZ+Z–e2/4πεor + B/rn
Need to find two new quantities: B and n.
Evaluate B at equilbrium because ∂Elat/∂r = 0 at r = ro (ro is the equilibrium internuclear distance measured experimentally)
∂Elat/∂r = –N0MZ+Z–e2/4πεor2 – nB/rn+1 = 0 at r = ro
Thus, nB/ron+1 = –N0MZ+Z–e2/4πεoro2
B = –N0MZ+Z–e2ron+1/4πεonro2 = –N0MZ+Z–e2ron–1/4πεon
So, at r = ro
Elat = N0MZ+Z–e2/4πεor + B/rn = N0MZ+Z–e2/4πεoro + (–N0MZ+Z–e2ron–1/4πεon)/ron
Elat = N0MZ+Z–e2/4πεoro – N0MZ+Z–e2/4πεonro
Elat = N0MZ+Z–e2(1 – 1/n)/4πεoro
To find n, we must move away from the equilibrium position. The slope of the energy/distance curve on the low r side is primarily determined by n in the repulsion term. Experimentally, this can be obtained by compressing the sample, i.e. measuring the compressibility, κ:
κ = –(1/Vo)(∂V/∂P)T (Vo = equilibrium molar volume; V = volume, P = pressure, T = temperature)
It can be shown that κ = (4πεo)(18ro4)/Me2(n – 1)
Compressibilities in ionic compounds are quite small:
Another common approach term is using the Born-Mayer repulsion: Erep = Ae–d*/r
Using the same approach as above this gives: Elat = N0MZ+Z–e2(1 – d*/ro)/4πεoro
d* is typically assigned a value of 34.5 pm
4πεo = 1.113×10–10 J–1C2m–1
e = 1.602×10–19 C
M = 1.74756 (NaCl structure)
d = 5.628×10–10 m giving ro = 2.814×10–10 m
κ = 4.18×10–11 N–1m2
n is found by
n = 1 + (4πεo)(18ro4)/Me2κ = 1 + (1.113×10–10 J–1C2m–1)(18×(2.814×10–10 m)4/ (1.74756)(1.602×10–19 C)2(4.18×10–11 N–1m2)
n = 1 + 6.70 J–1m3/N–1m2 = 1 + 6.70 N·m/J = 7.70 (N·m = J)
Elat = N0MZ+Z–e2(1 – 1/n)/4πεoro = (6.022×1023 mol–1)(1.74756)(+1)(–1)(1.602×10–19 C)2(1 – 1/7.70)/ (1.113×10–10 J–1C2m–1)(2.814×10–10 m)
Elat = –750348 J/mol = –750.3 kJ/mol
This compares to 769.4 kJ/mole experimental (2.5% error)
lattice energies are thermodynamically negative but reported as positive values so this is typically reported as 750.3 kJ/mol
Using the Born-Mayer equation gives Elat = 756.6 kJ/mol
Kapustinskii deduced that the Madelung constants were approximately correlated with the number of ions in the repeat unit. This led him to propose a lattice energy formula that does not need knowledge of the crystal structure:
Elat = –1202νZ+Z–[1 – 0.345/(r+ + r–)]/ (r+ + r–) where ν is the total number of ions in the repeat unit of the ionic compound and r+ and r– are the cationic and anionic radii, respectively, in units of Å giving Elat in units of kJ/mol
Note that r+ + r– = ro
For NaCl, ν = 2 and r+ + r– = 2.814 Å
Elat = –1202(2)(+1)(–1)[1 – 0.345/2.814]/2.814 = 741.6 kJ/mol