Wave properties of electrons are constrained by certain boundary conditions:
1) the electron exists somewhere in space
2) the electron is continuous
3) the electron is finite
When these conditions are imposed upon Schroedinger's equation, the result is quantization:
1) each electron can only have certain energies (energy quantization)
2) each electron can only occupy certain volumes of space (spatial quantization)
Hydrogenic Wavefunctions:
The Hamiltonian is solved in radial coordinates giving energies and wavefunctions
Ψ = R(r)×Y(θ,φ)
quantization is described by a set of numbers called quantum numbers:
n = principal quantum number, found only in R, distance dependence
l = orbital angular momentum, found in R and Y, orbital shape
ml = magnetic quantum number, found only in Y, orientation in space
The quantum numbers are mathematically related
n = 1, 2, 3, 4, ...
l = n–1, n–2, n–3, ..., 0
ml = –l, –l+1, –l+2, ..., l–2, l–1, l
The l quantum number is usually designated by a letter:
lletter
0s
1p
2d
3f
4g
Experimental evidence (and relativistic theory) indicates the presence of a fourth quantum
number, ms, the spin quantum number = ±1/2
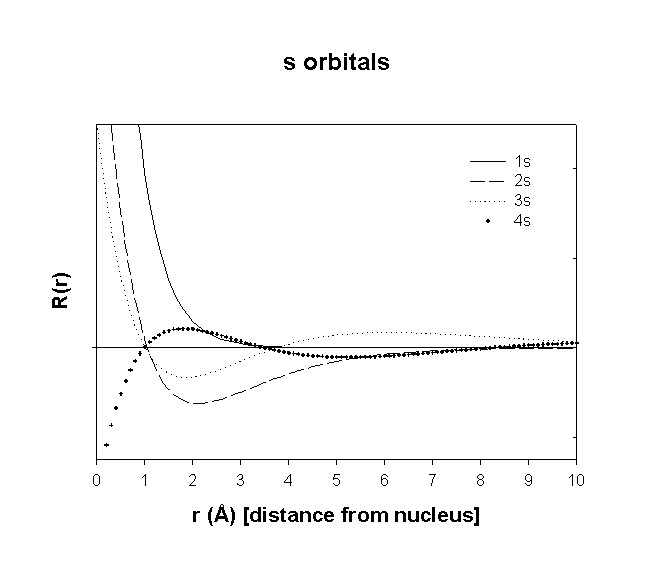
Radial Wavefunctions:

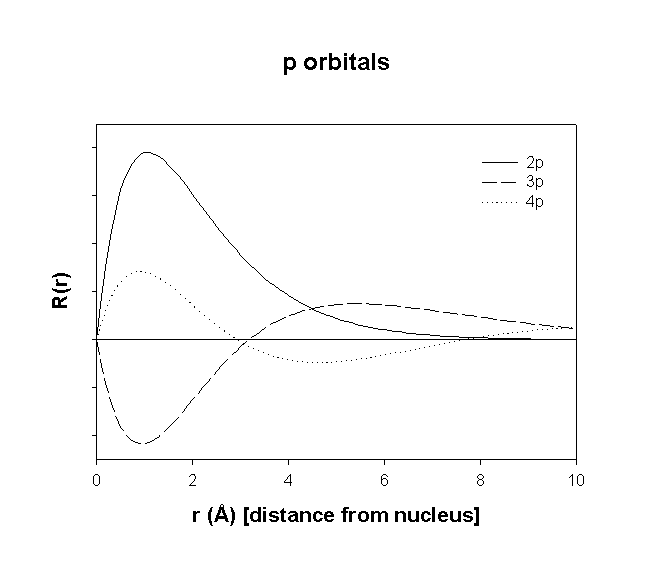
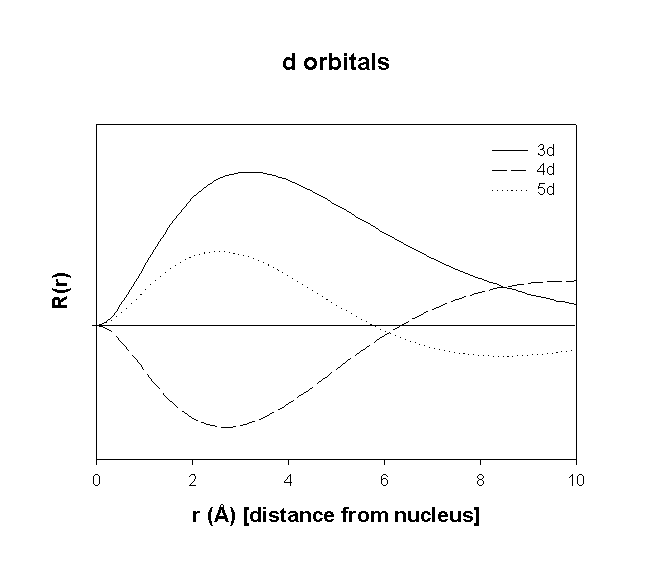
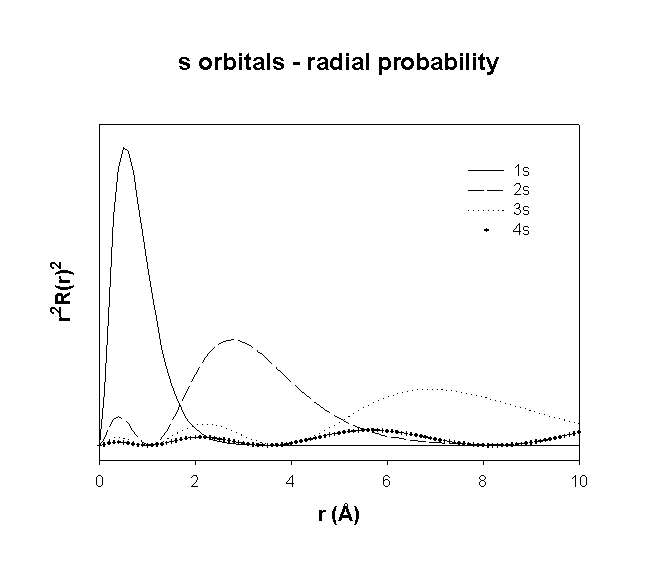
Angular Wavefunctions:
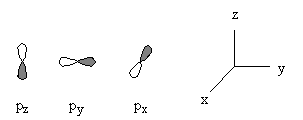
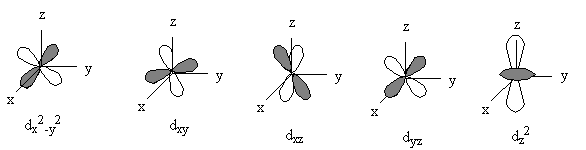
General nodal properties:
total number of nodes = n–1
total number of planar (or angular) nodes = l
total number of radial nodes = n–l–1
The Schroedinger equation can not be solved exactly so assumptions must be made; assume the hydrogenic obitals are adequate and electrons occupy them in some fashion
Two guiding principals used to account for electron configurations
Aufbau Principle: electrons occupy orbitals in such a manner to give the lowest possible total energy
Pauli Exclusion Principle: each electron in an atom is described by a unique set of quantum numbers (n, l, ml, ms)
The Periodic Table is based on electron configurations and can be used to predict them but not absolute (electron configurations are experimental quantities)
Aids in finding correct electron configurations from the Periodic Table
Half-filled phenomenon: when d or f electrons are the valence shell, if a shift of 1 electron (occasionally 2 but this is not predictive) from an s orbital to the d or f orbital leads to a filled or half filled d or f orbital, this will stabilize the electron configuration.
anions: add electrons to the neutral atom and follow above rules.
cations: electrons are always removed from the orbitals with the largest principal quantum number (n); the remaining electrons fill the orbitals with the lowest n consistent with the Pauli Principle electron configurations denote n and l quantum numbers